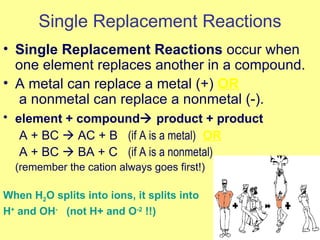
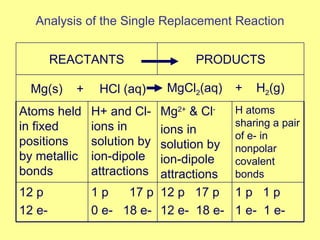
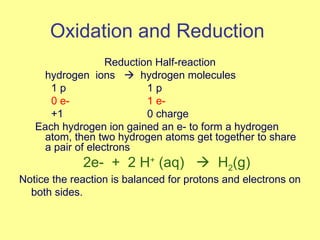
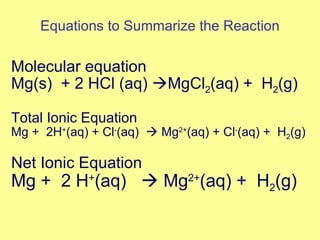
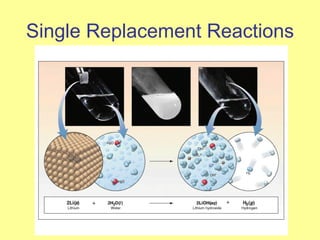
Magnesium reacted immediately with hydrochloric acid, bubbling rapidly until fully dissolved. Zinc reacted more slowly with bubbling. Aluminum reacted slowly only with the stronger acid. Copper did not react at all. This is because magnesium, zinc and aluminum are more reactive than hydrogen in the acid, replacing it to form magnesium chloride, hydrogen gas and electrons in a single replacement reaction. Copper is less reactive than hydrogen and did not replace it.