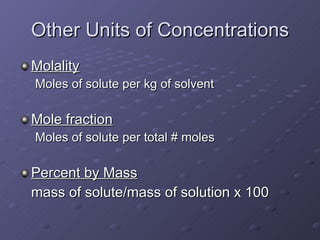
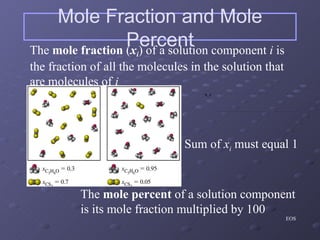
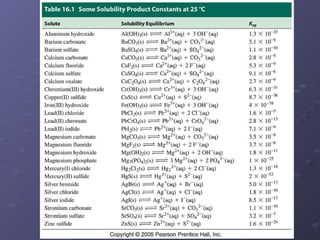
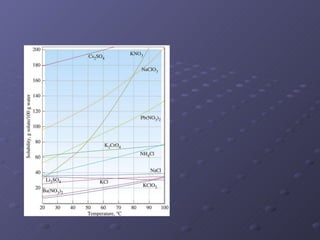
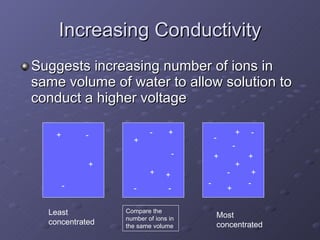
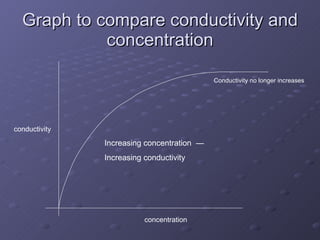
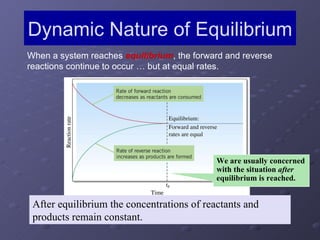
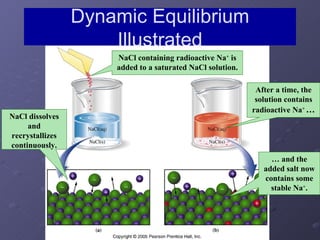
This lab experiment investigated how the concentration and conductivity of a sodium chloride solution changed as sodium chloride was added incrementally. The solution's conductivity increased with each addition as the number of ions increased, until the fifth addition when undissolved salt remained, indicating saturation. The goal was to prepare saturated solutions of various ionic compounds and determine their concentrations using Ksp calculations and measurements.




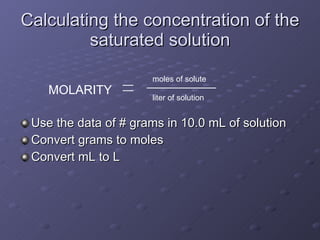



![Ksp- the equilibrium constant for saturated solutions of ionic compounds NaCl(s) Na + (aq) + Cl - (aq) Ksp = [Na + ][Cl - ] If the concentration of dissolved NaCl in a saturated solution is 5 M Then the concentration of sodium ions must be 5 M and the concentration of chloride ions must be 5 M Ksp = [Na + ][Cl - ] = (5 M)(5M) = 25](https://image.slidesharecdn.com/lab3-091106220017-phpapp02/85/Lab-3-14-320.jpg)
![Calculating Concentration given the Ksp AgCl(s) Ag + (aq) + Cl - (aq) Ksp = [Ag + ][Cl - ] The concentration is unknown Let x = [AgCl], then x also x = [Ag + ] and x = [Cl - ] Ksp = [Ag + ][Cl - ] = (x)(x) = x 2 = 1.6 x 10 -10 So x = 1.3 x 10 -5 M Much less silver chloride is present in saturated solution than sodium chloride The lower Ksp shows the compound is less soluble](https://image.slidesharecdn.com/lab3-091106220017-phpapp02/85/Lab-3-15-320.jpg)
![Given the Concentration calculate the Ksp Ag 2 S(s) 2Ag + (aq) + S 2- (aq) Ksp = [Ag + ] 2 [S 2- ] The concentration of silver sulfide in a saturated solution is 3.1 x 10 -17 M = [Ag 2 S] The concentration of sulfide ions must be 3.1 x 10 -17 M = [S 2- ] The concentration of silver ions must be 2 x 3.1 x 10 -17 M = 6.2 x10 -17 M = [Ag + ] Ksp = [Ag + ] 2 [S 2- ] = (6.2 x10 -17 ) 2 (3.1 x 10 -17 ) = 3.2 x 10 -49 A very small Ksp indicates that this compound is barely soluble](https://image.slidesharecdn.com/lab3-091106220017-phpapp02/85/Lab-3-16-320.jpg)

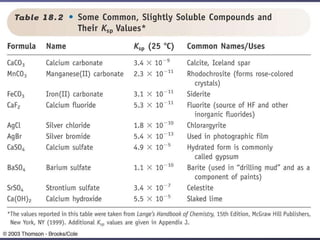
![Calculating the Concentration of Calcium fluoride in a saturated solution Ksp = 5.3 x 10-11 Reversible reaction at equilibrium CaF 2 (s) Ca 2+ (aq) + 2F - (aq) Ksp = [Ca 2+ ][F - ] 2 If x = [CaF 2 ], then x = [Ca 2+ ] and 2x = [F - ] Ksp = [Ca 2+ ][F - ] 2 = 5.3 x 10 -11 = (x)(2x) 2 (x)(4x 2 ) = 4x 3 = 5.3 x 10 -11 Divide by 4 and then cube root](https://image.slidesharecdn.com/lab3-091106220017-phpapp02/85/Lab-3-19-320.jpg)