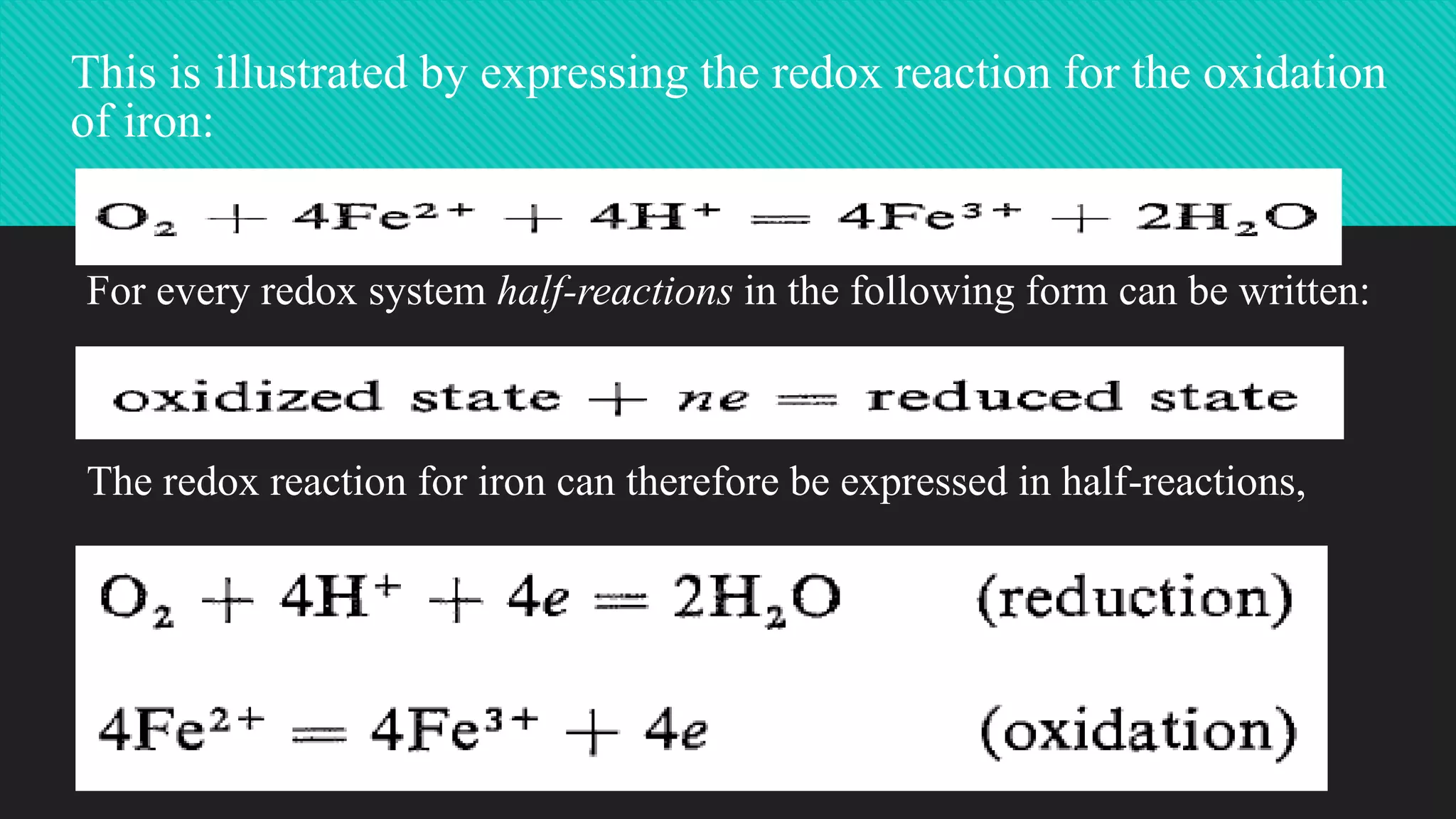
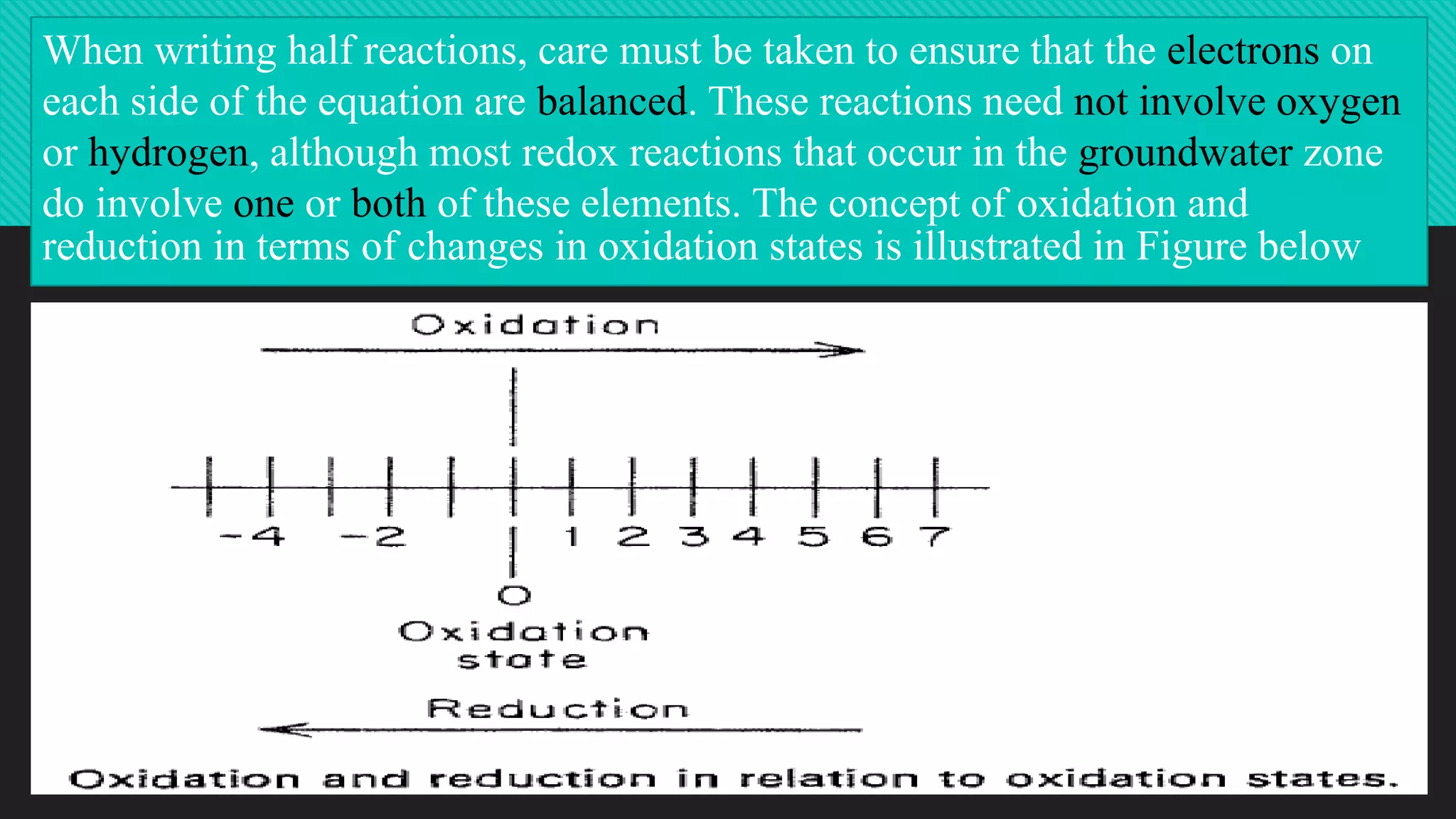
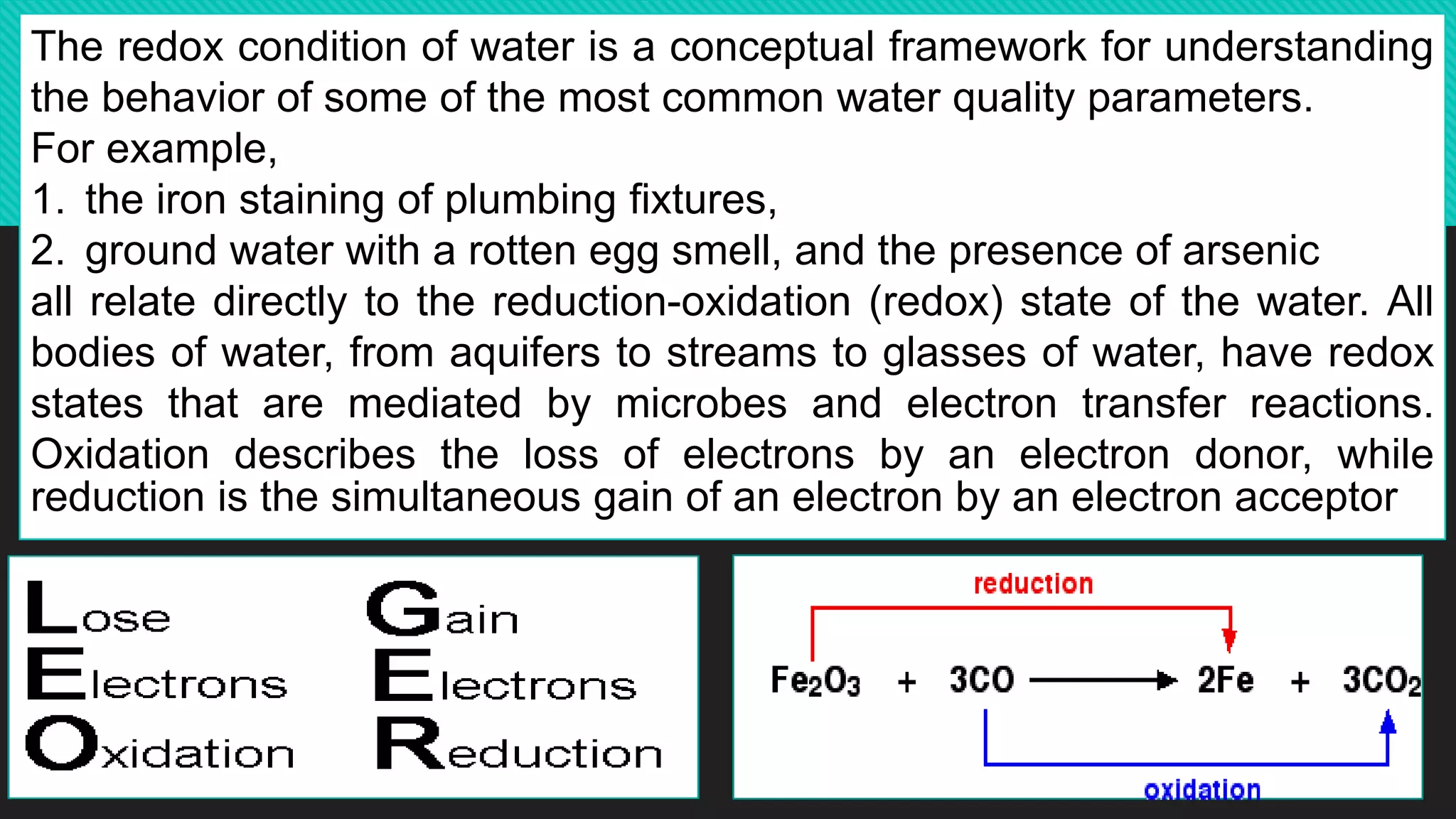
The document discusses oxidation-reduction (redox) reactions, highlighting that every oxidation is accompanied by a corresponding reduction, maintaining electron balance. It explains the role of redox states in groundwater quality, detailing how electron transfer mediated by microorganisms affects the behavior of water constituents like metals and contaminants. The impact of leachate from landfills on groundwater redox conditions is emphasized, illustrating how older landfills can introduce reducing conditions into local water systems.