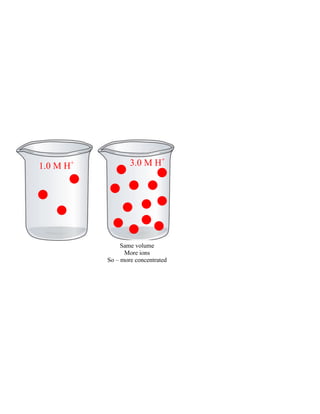
The document describes a lab experiment comparing the reactions of different metals with hydrochloric acid. Students observed the reactions of zinc, magnesium, copper, and aluminum with HCl of varying concentrations. Zinc and magnesium reacted vigorously while copper did not react at all. The purpose was to model oxidation-reduction reactions and construct equations to summarize the single replacement reactions. Students analyzed the reactions in terms of atoms becoming ions, oxidation states changing, and the energy changes involved. Reactions that produced heat were determined to be exothermic.