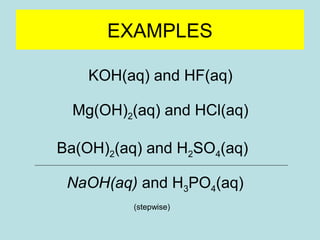
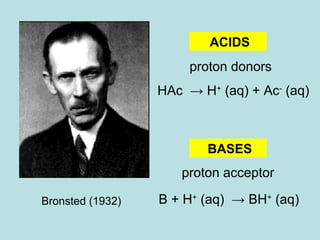
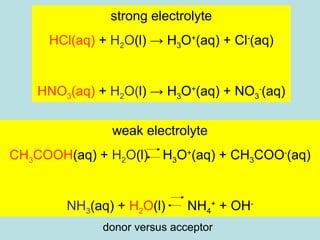
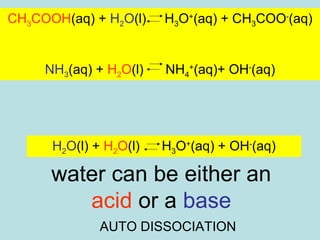
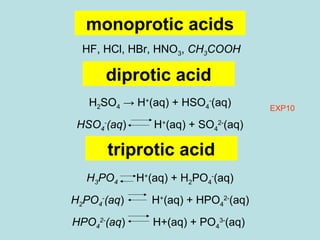
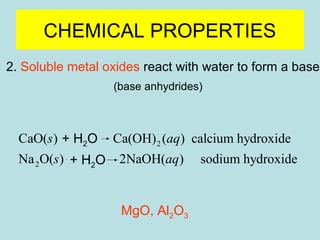
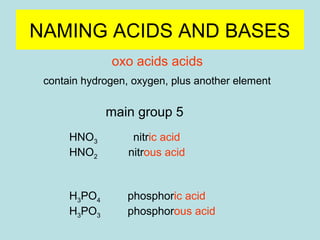
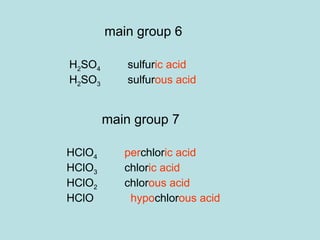
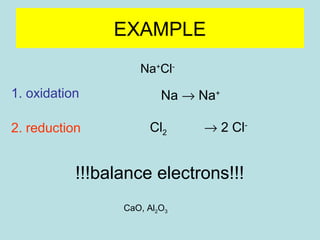
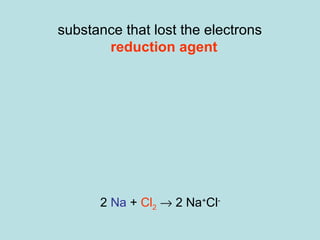
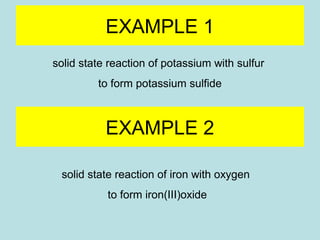
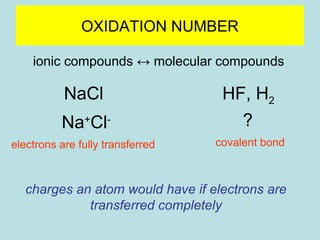
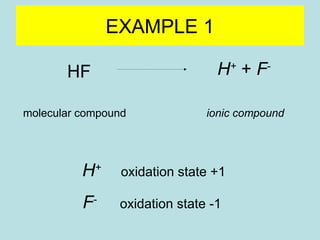
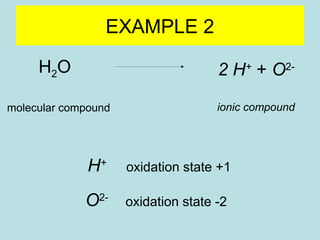
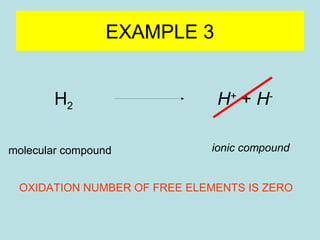
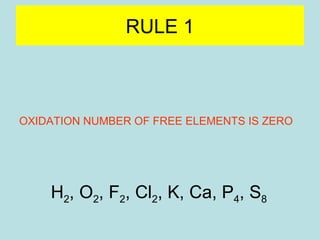
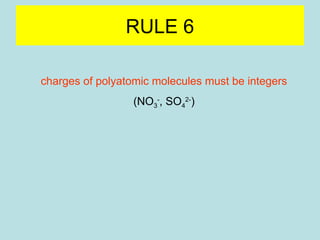
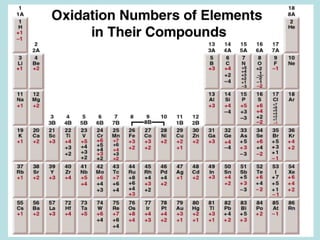
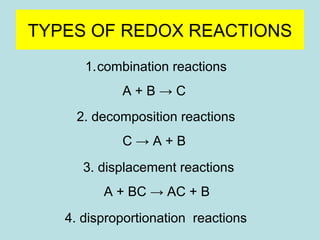
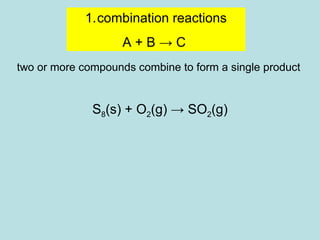
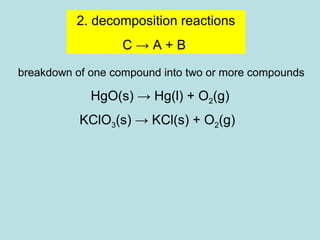
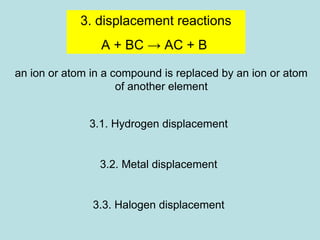
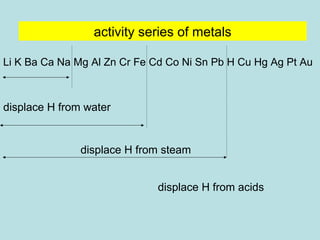
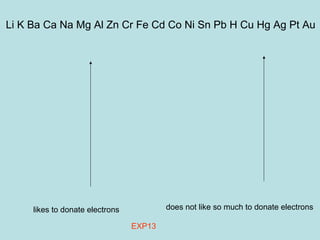
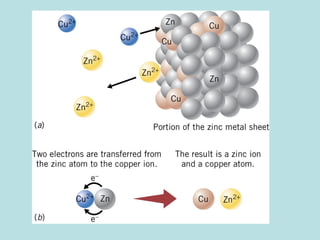
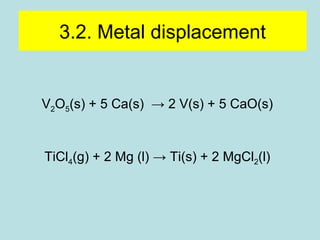
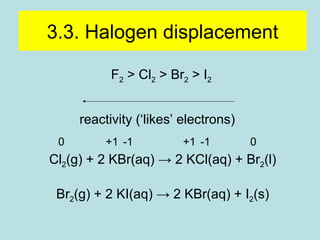
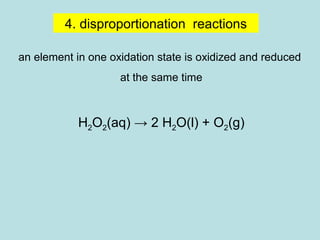
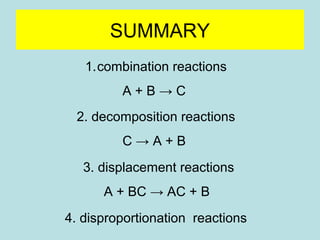
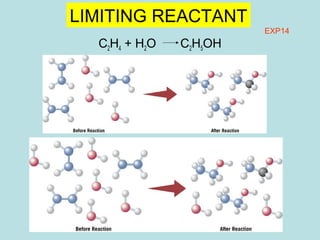
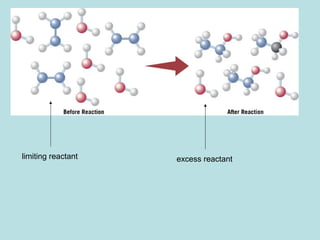
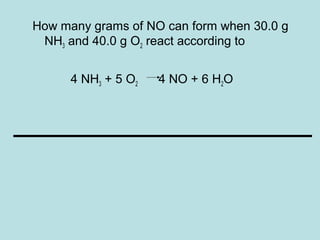
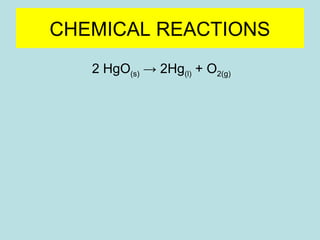
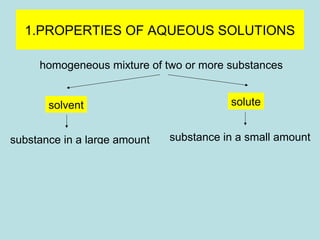
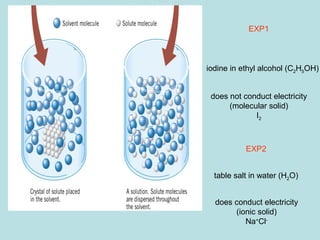
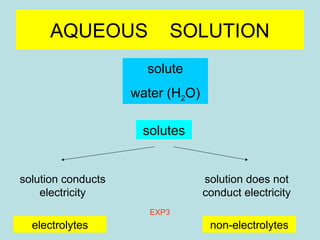
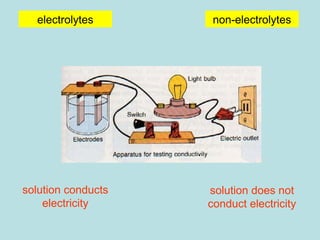
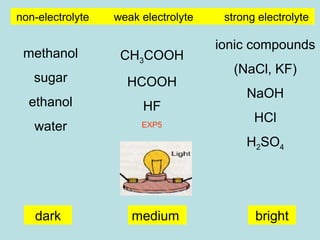
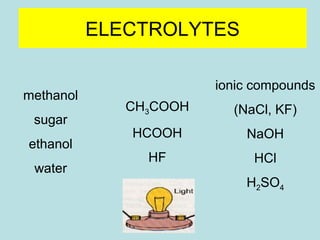
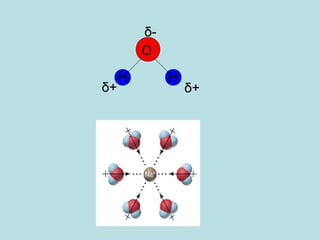
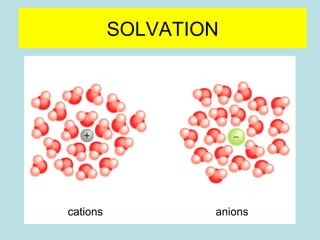
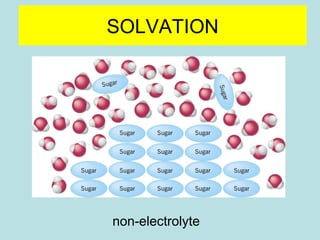
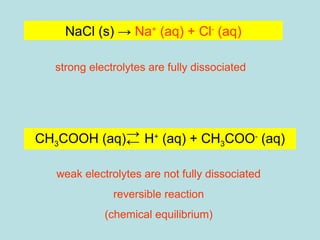
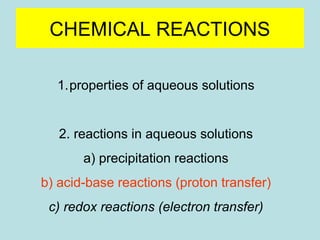
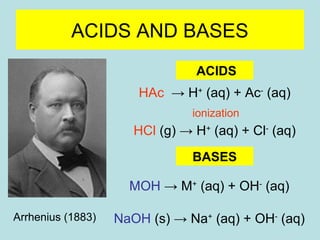
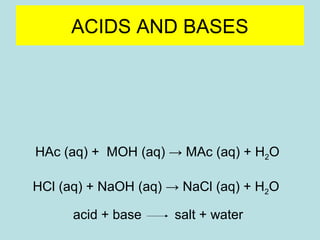
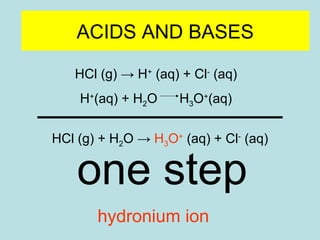
This document provides an overview of chapter 4 from a chemistry textbook, which covers chemical reactions. It begins by defining electrolytes, non-electrolytes, and discussing the properties of aqueous solutions. It then covers the three main types of reactions that occur in aqueous solutions: precipitation reactions, acid-base reactions involving proton transfer, and redox reactions involving electron transfer. Specific examples of each reaction type are provided. Key concepts around oxidation, reduction, and oxidation numbers are also explained.

![SOLUTION
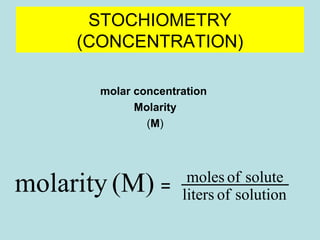
percentage concentration
% = g [solute] / g solvent X 100
12 g of sodium chloride are solved in 150 g of water.
Calculate the percentage concentration
8 %](https://image.slidesharecdn.com/chapter4tro-141027222038-conversion-gate02/85/Elektrolit-dan-Nonelek-9-320.jpg)






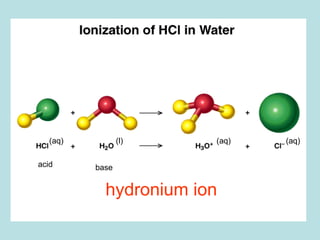
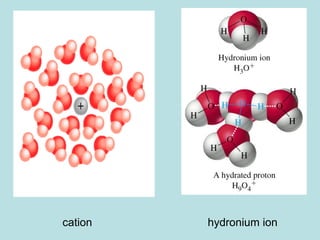
![PROPERTIES OF ACIDS
1. acids have a sour taste
vinegar – acetic acid
lemons – citric acid
2. acids react with some metals to form hydrogen
2 HCl(aq) + Mg(s) → MgCl2(aq) + H2(g)
3. acids react with carbonates to water and carbon dioxide
2 HCl(aq) + CaCO3(s) → CaCl2(aq) + [H2CO3]
H2CO3 → H2O(l) + CO2(g)
EXP8
EXP9
4. some acids are hygroscopic
H2SO4 (conc)](https://image.slidesharecdn.com/chapter4tro-141027222038-conversion-gate02/85/Elektrolit-dan-Nonelek-36-320.jpg)