Embed presentation
Download to read offline

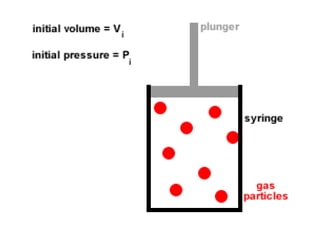
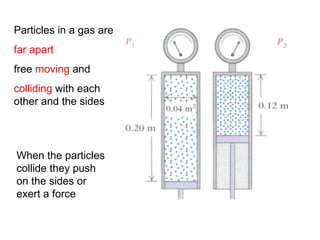
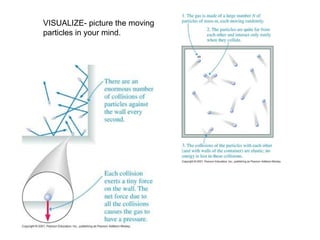
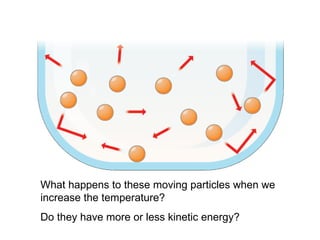
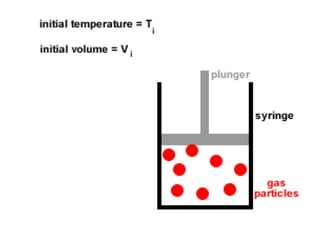
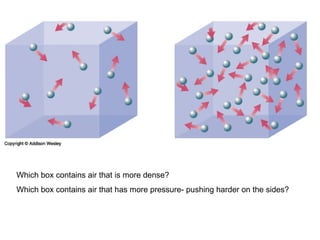


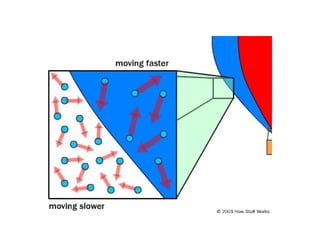
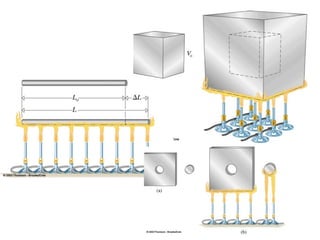
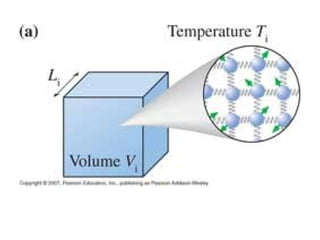
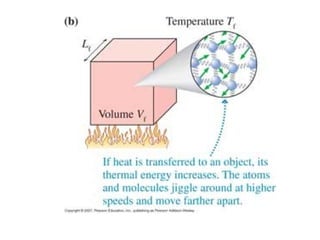
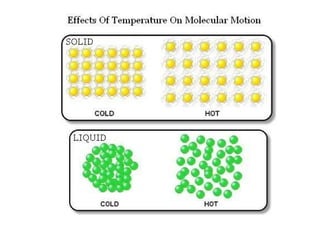
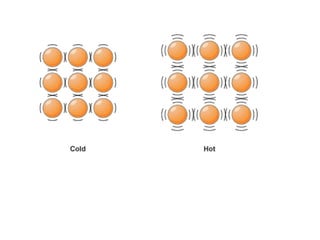

The document discusses how increasing the temperature of gas particles affects their kinetic energy, density, and pressure. Heating gas particles gives them more kinetic energy, lowering their density and increasing the pressure they exert on container walls. Heating trapped air particles expands the volume they occupy by speeding up the particles' motion, while heating the air in a hot air balloon makes it less dense than the surrounding air, enabling it to float.















