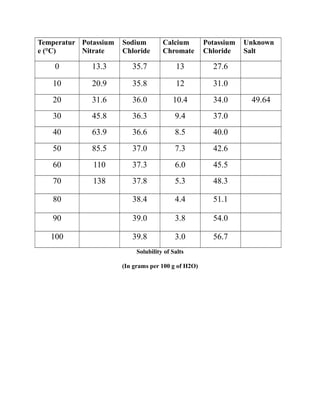
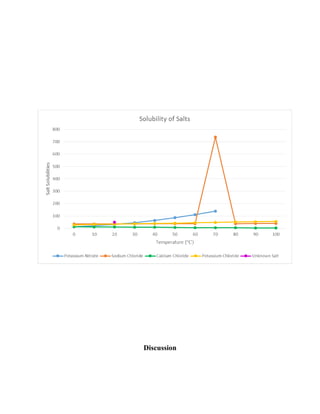
The document summarizes a lab experiment on determining the solubility of an unknown salt. Students found the solubility to be 66.28g/100g H2O initially but made errors. Another student correctly obtained 49.64g/100g H2O for potassium chloride, which has an actual solubility of 34.0g/100g H2O at 20°C. Errors included insufficient salt dissolution and not properly weighing materials. The purpose of determining a salt's solubility at different temperatures was achieved but the students' results were inaccurate due to mistakes made during the experiment.









![References
Dr. Breinan. (n.d). Solubility Lab. Retrieved from https://www.google.com/url?
sa=t&rct=j&q=&esrc=s&source=web&cd=6&ved=0CC8QFjAF&url=https%3A%2F
%2Fwww.glastonburyus.org%2Fstaff%2FBREINANH%2Fregular%2Ffirstlabs%2FDocuments
%2FSolubilityCurve.lab.student.doc&ei=Kw9dVPOFJM71yASR2oCIAQ&usg=AFQjCNEVwB
UXnXY8gkM5_F0LZoyO_Kylhg&sig2=jl1mdoxtlClxyIRy3c3vaw.
Solubility equilibria of salts Part 1: Solubility products and calculations. (n.d.). Retrieved
from http://www.chem1.com/acad/webtext/solut/solut-6a.html.
[Solubility of potassium chloride]. (n.d). Retrieved from
http://chemicals.etacude.com/p/more/kcl.html.](https://image.slidesharecdn.com/86c65232-dc2e-4f9f-b2b7-94c60760df4a-150416123630-conversion-gate01/85/Chem-Lab-Report-1-12-320.jpg)