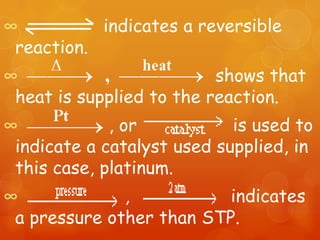
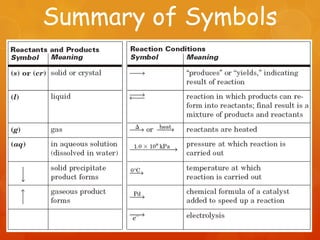
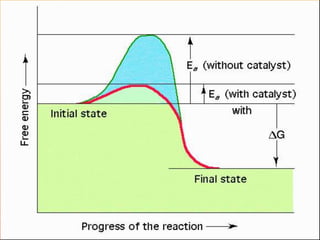
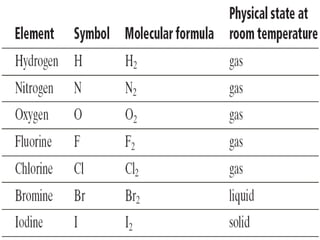
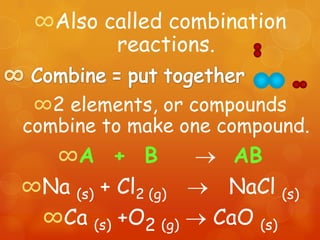
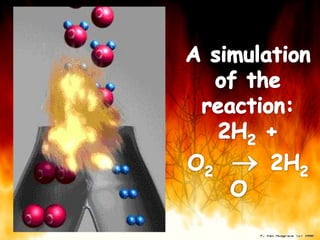
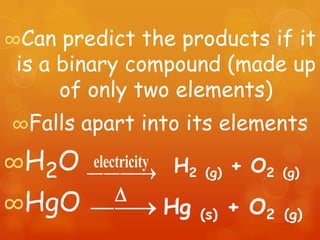
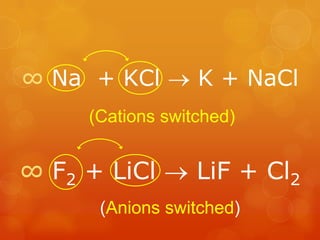
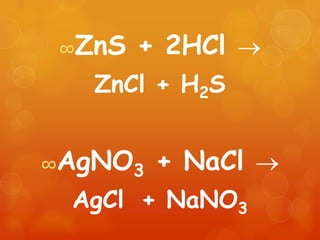
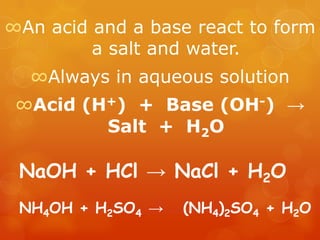
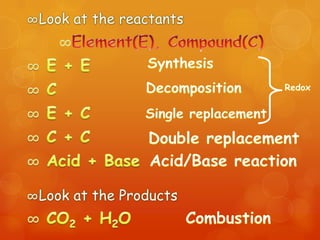
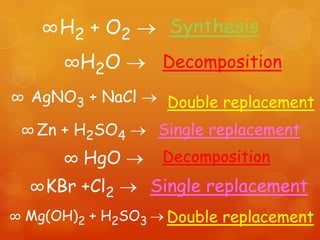
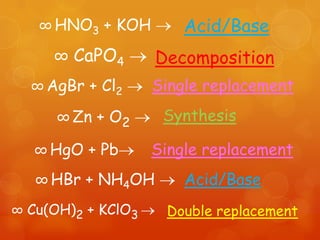
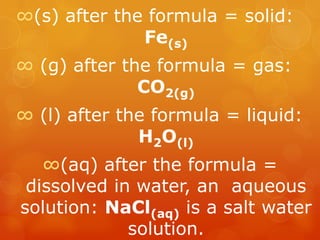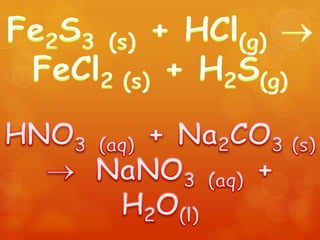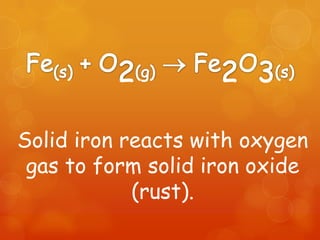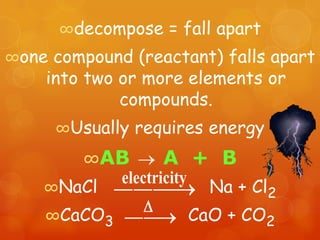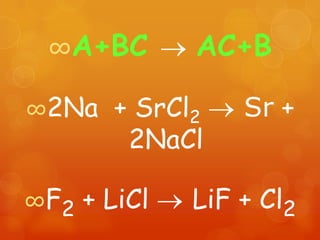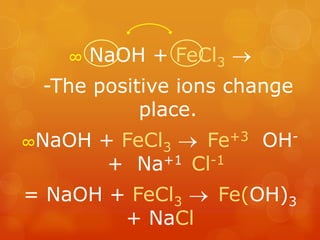The document provides information on how to write and interpret chemical equations. It defines reactants and products, explains the symbols used in equations, and describes the five main types of chemical reactions: synthesis, decomposition, single replacement, double replacement, and combustion.