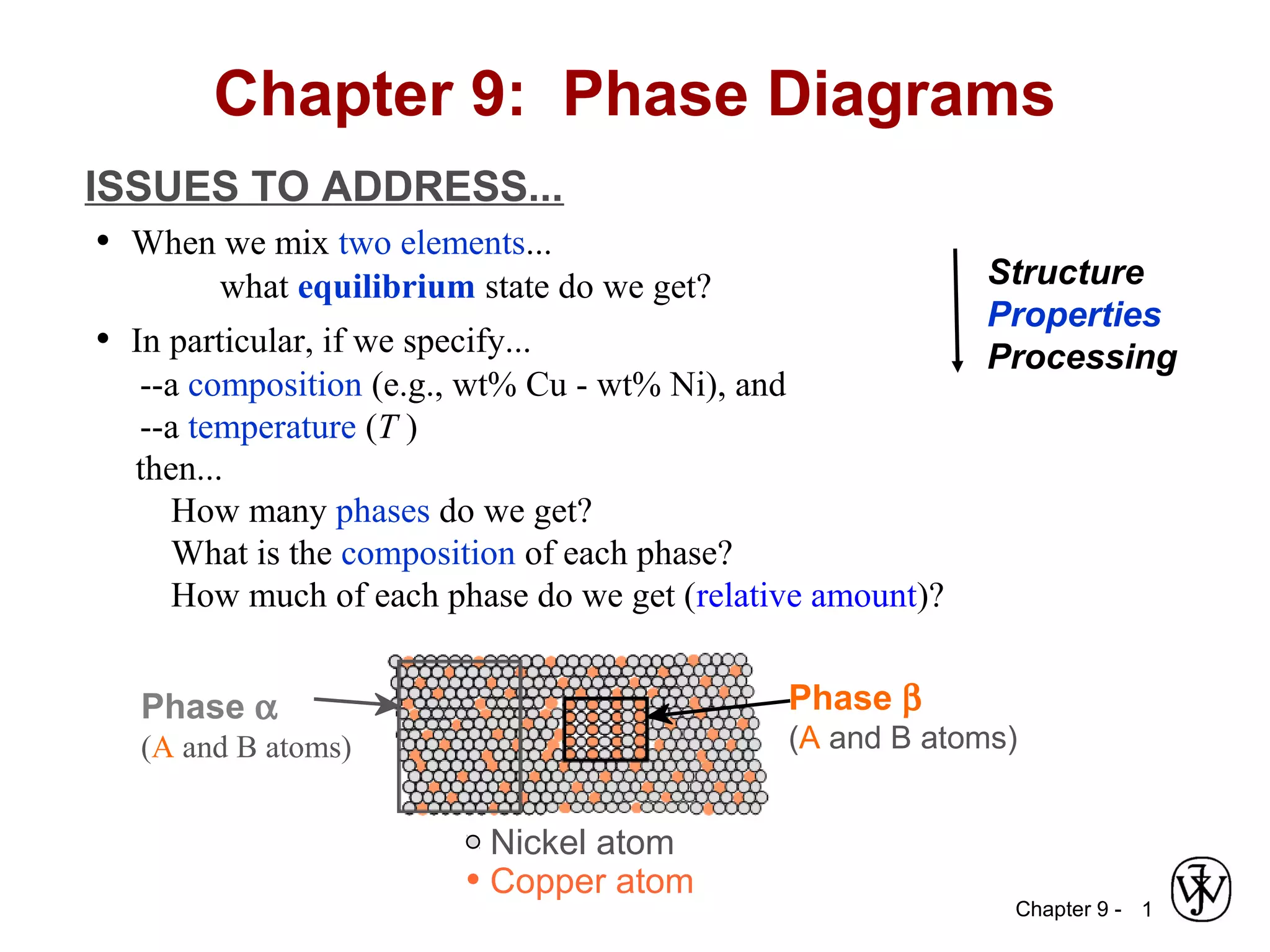
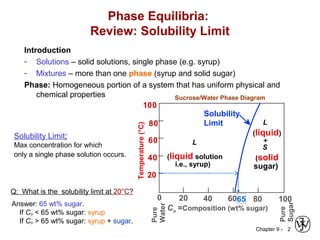
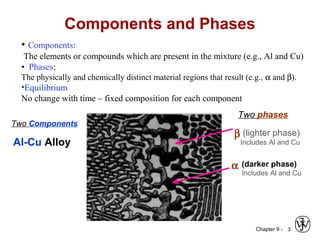
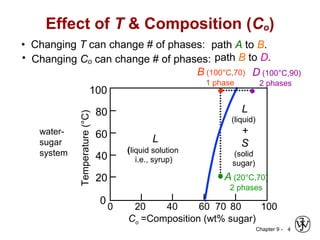
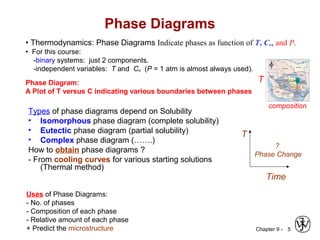
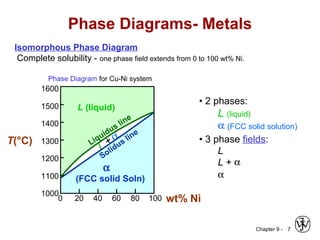
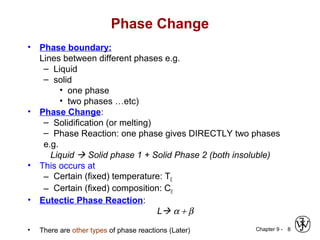
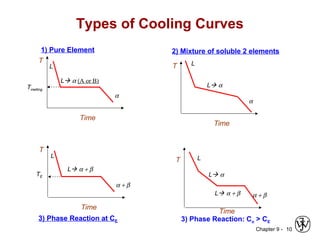
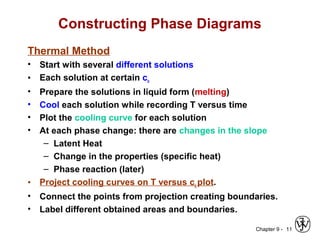
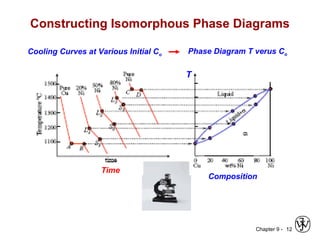
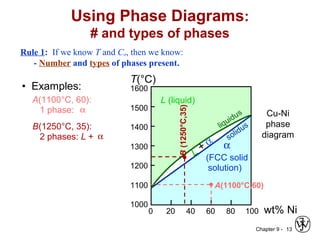
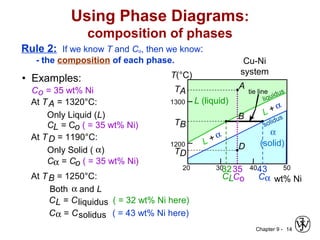
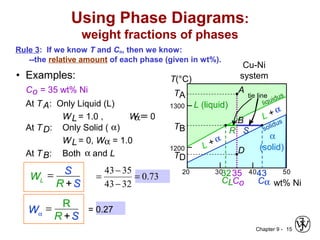
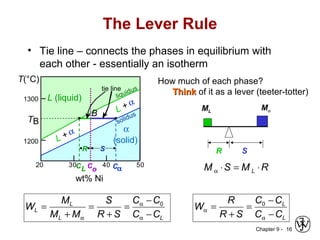
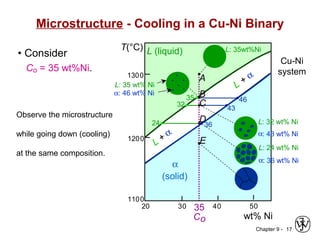
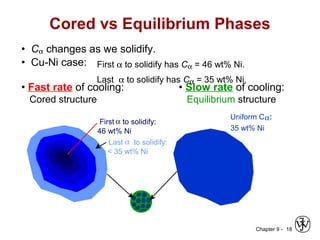
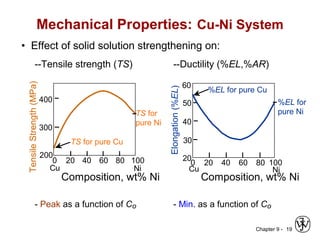
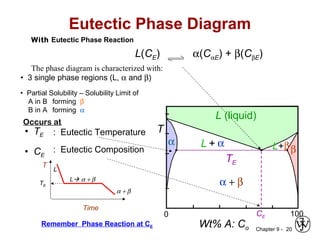
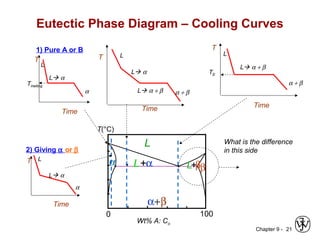
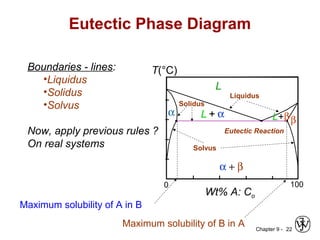
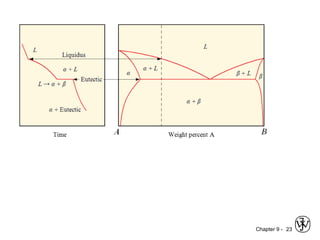
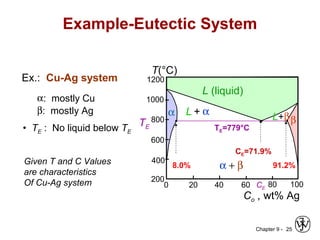
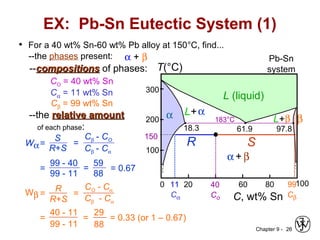
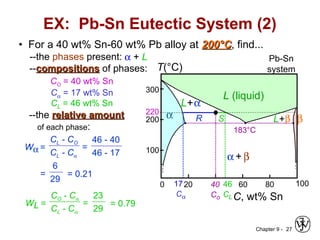
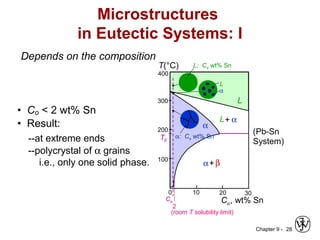
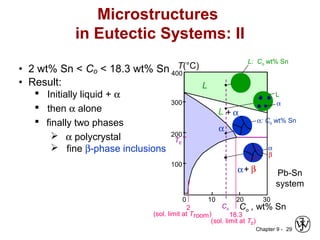
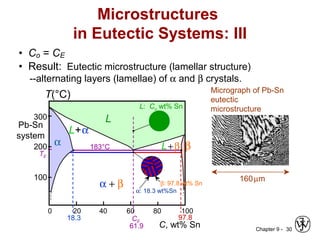
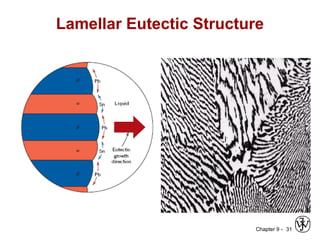
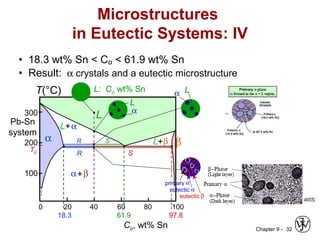
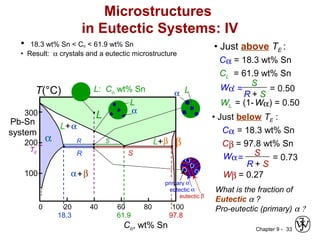
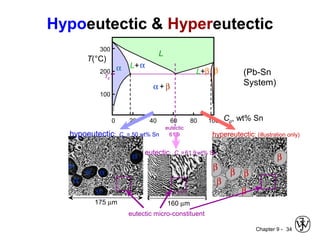
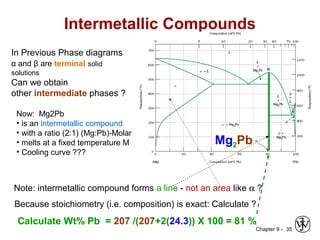
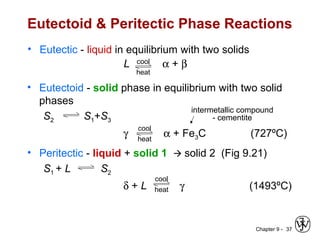
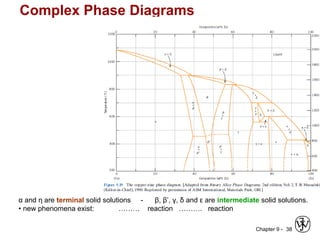
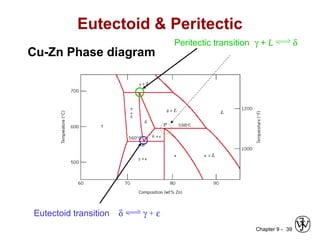
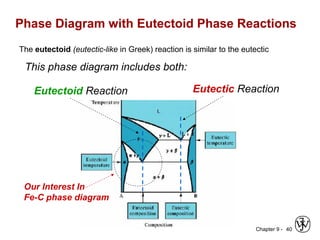
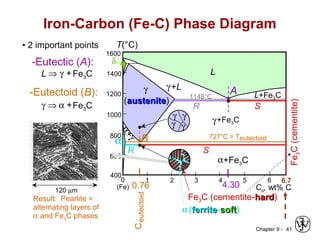
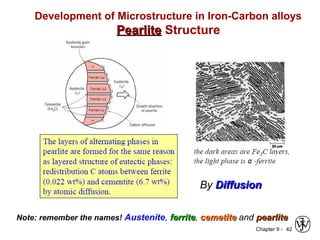
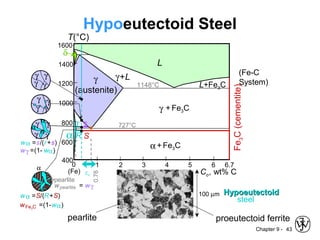
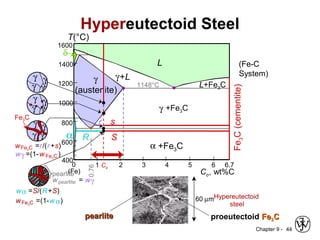
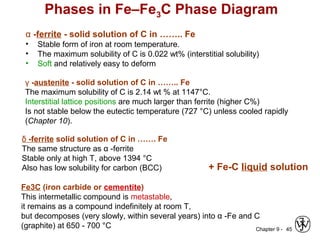
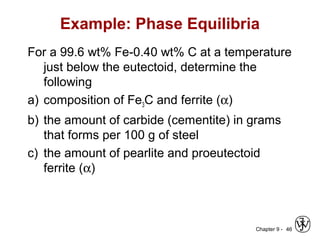
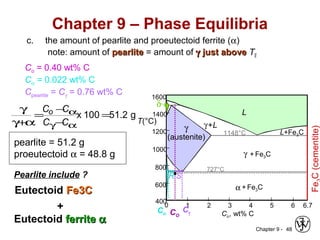
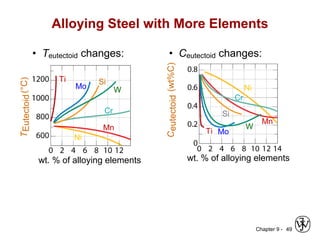
This document discusses phase diagrams and related concepts. It begins by asking questions about what equilibrium state and phases result from mixing two elements at a given composition and temperature. It then provides information on phase diagrams, including how they are constructed using cooling curve data from mixtures at different compositions. It discusses rules for determining phase information like number, composition, and amounts from a phase diagram given temperature and composition. Finally, it gives examples of an isomorphous system and eutectic system phase diagram.