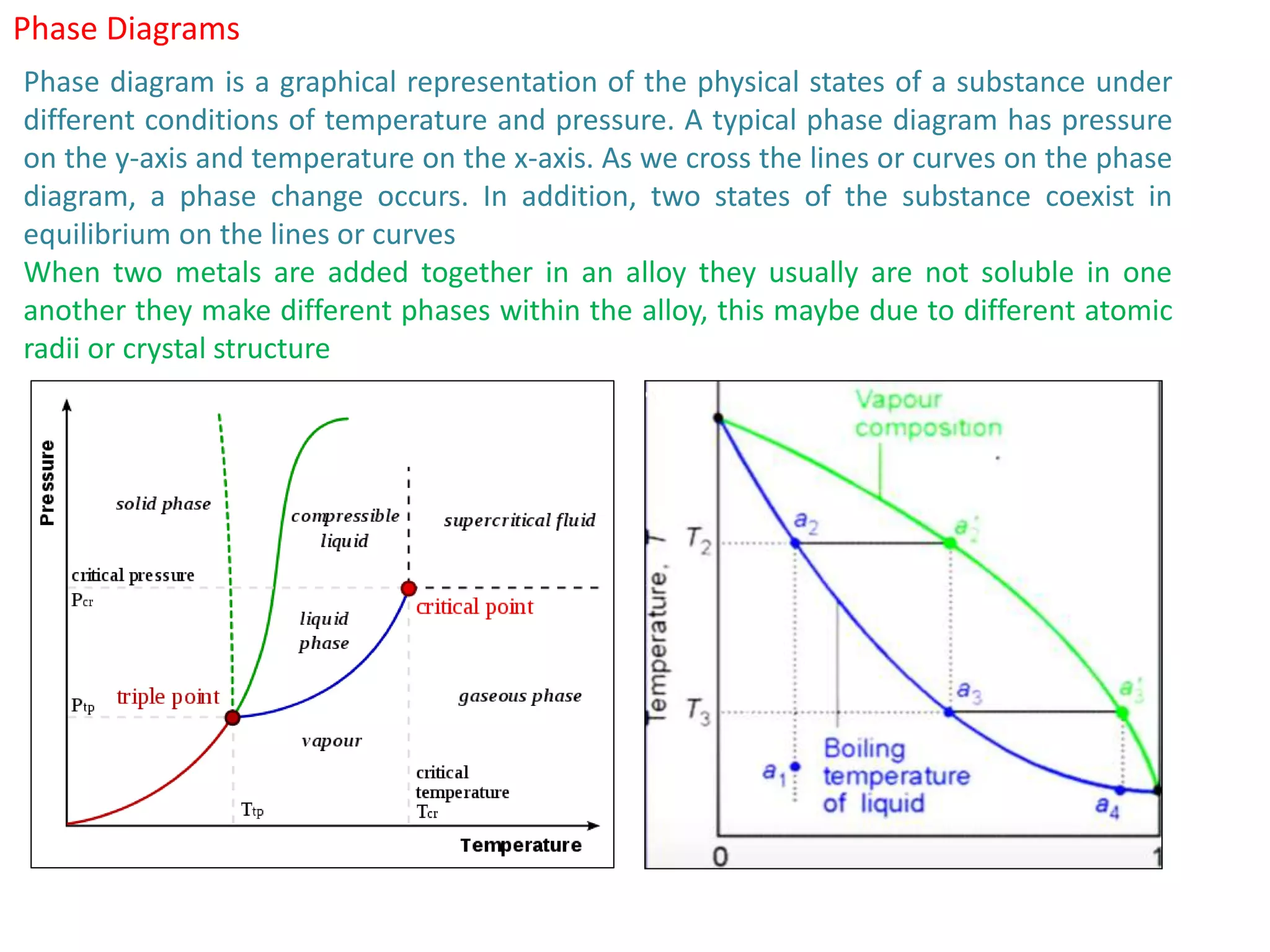
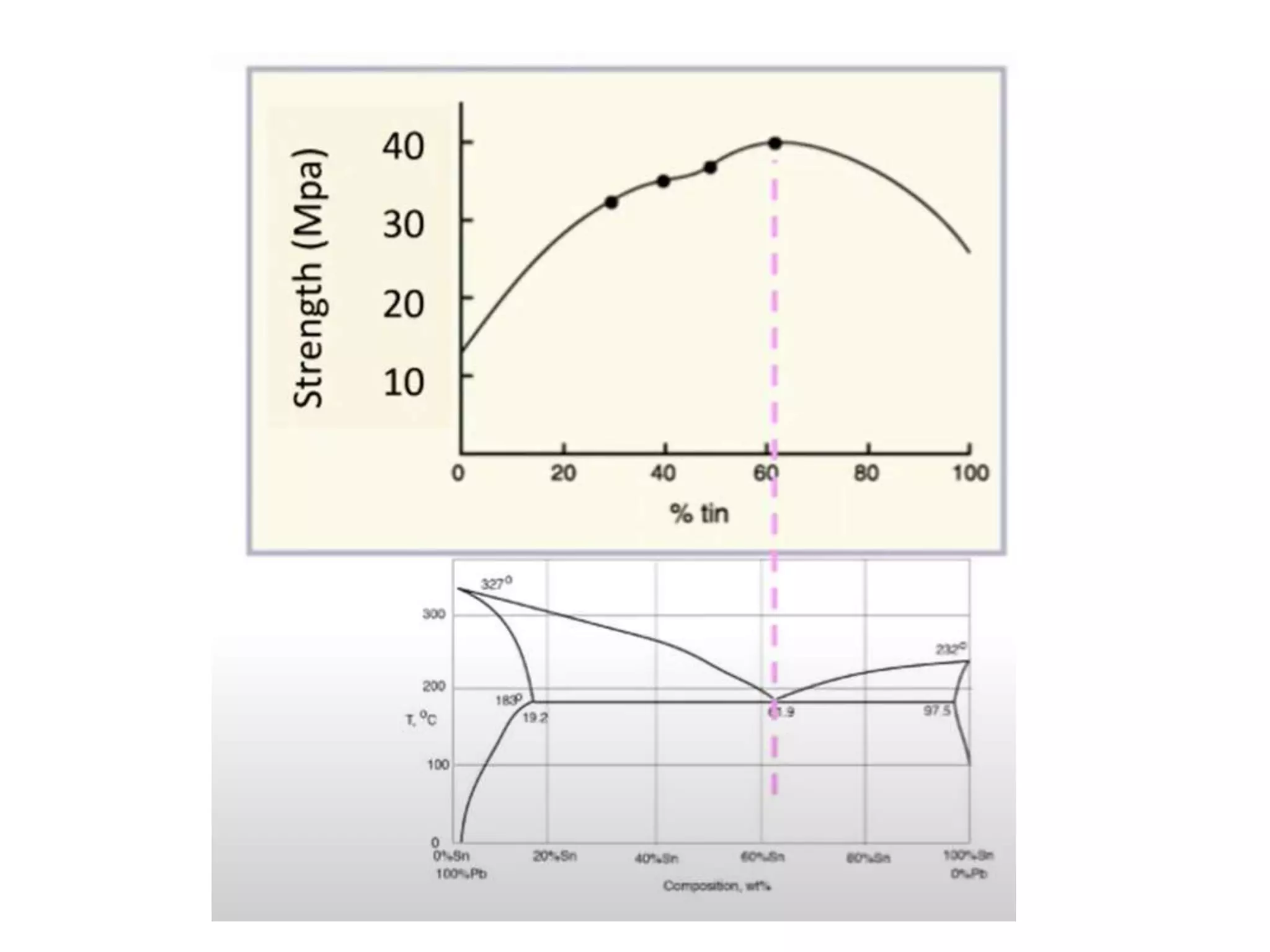
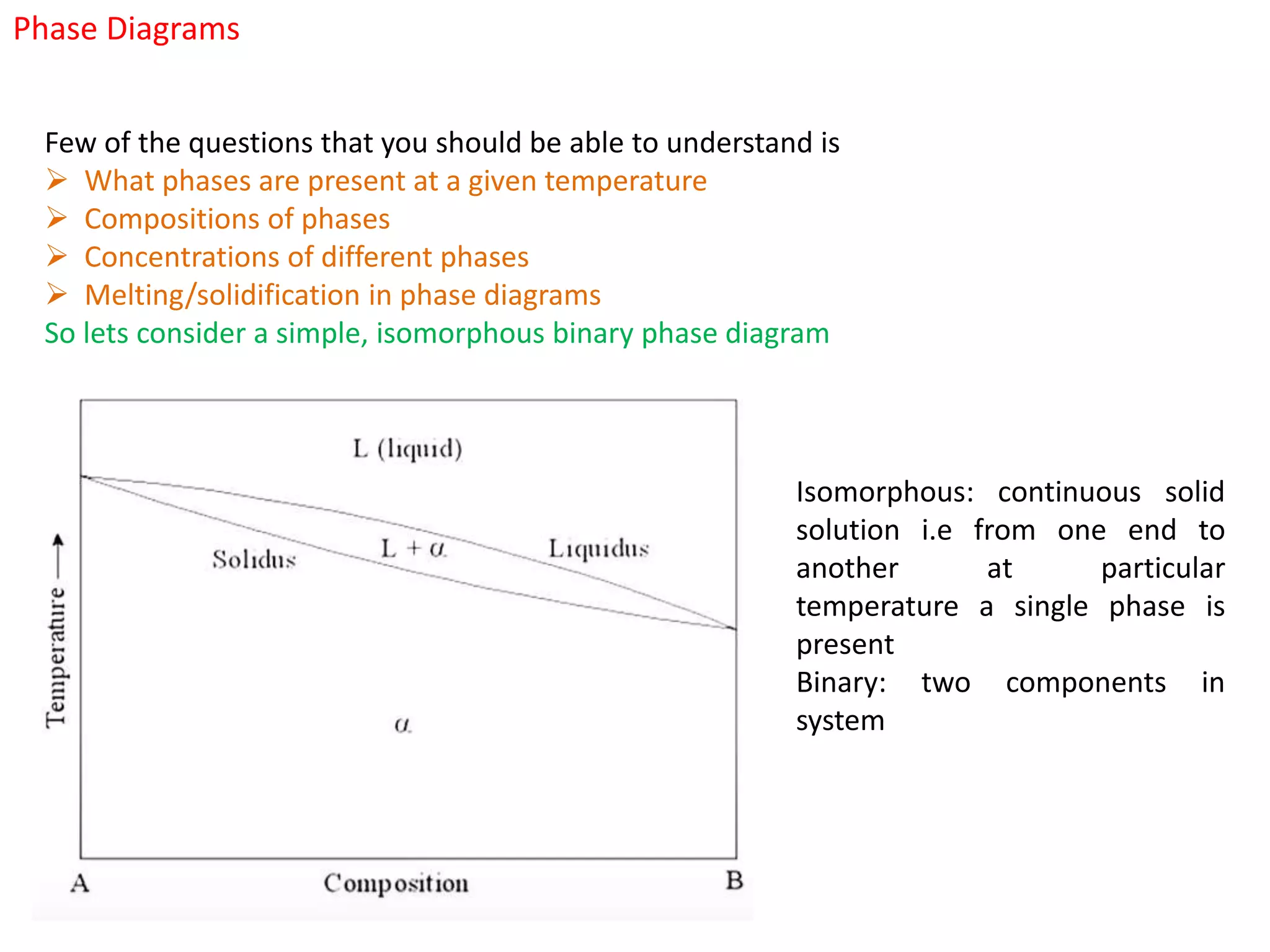
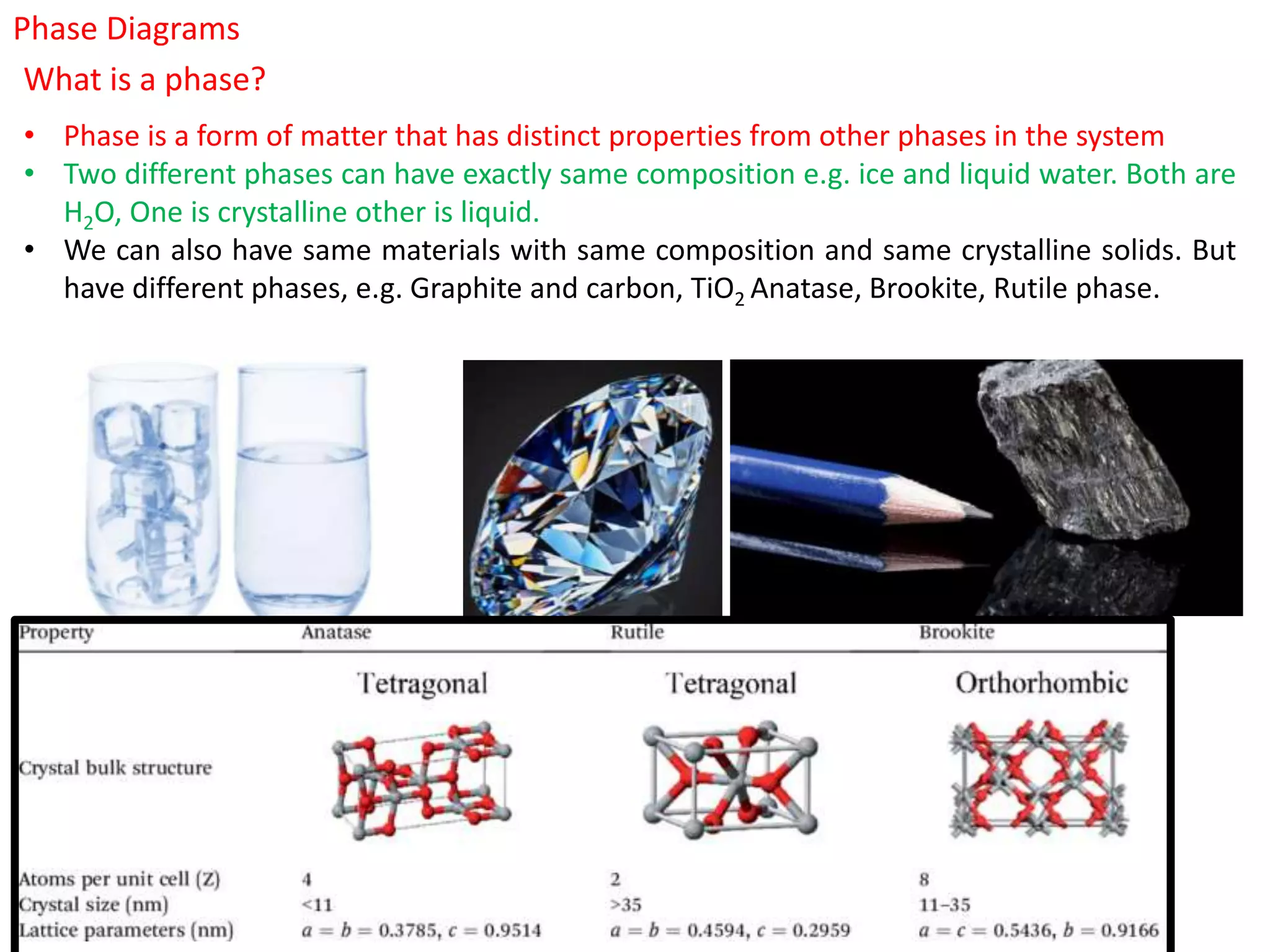
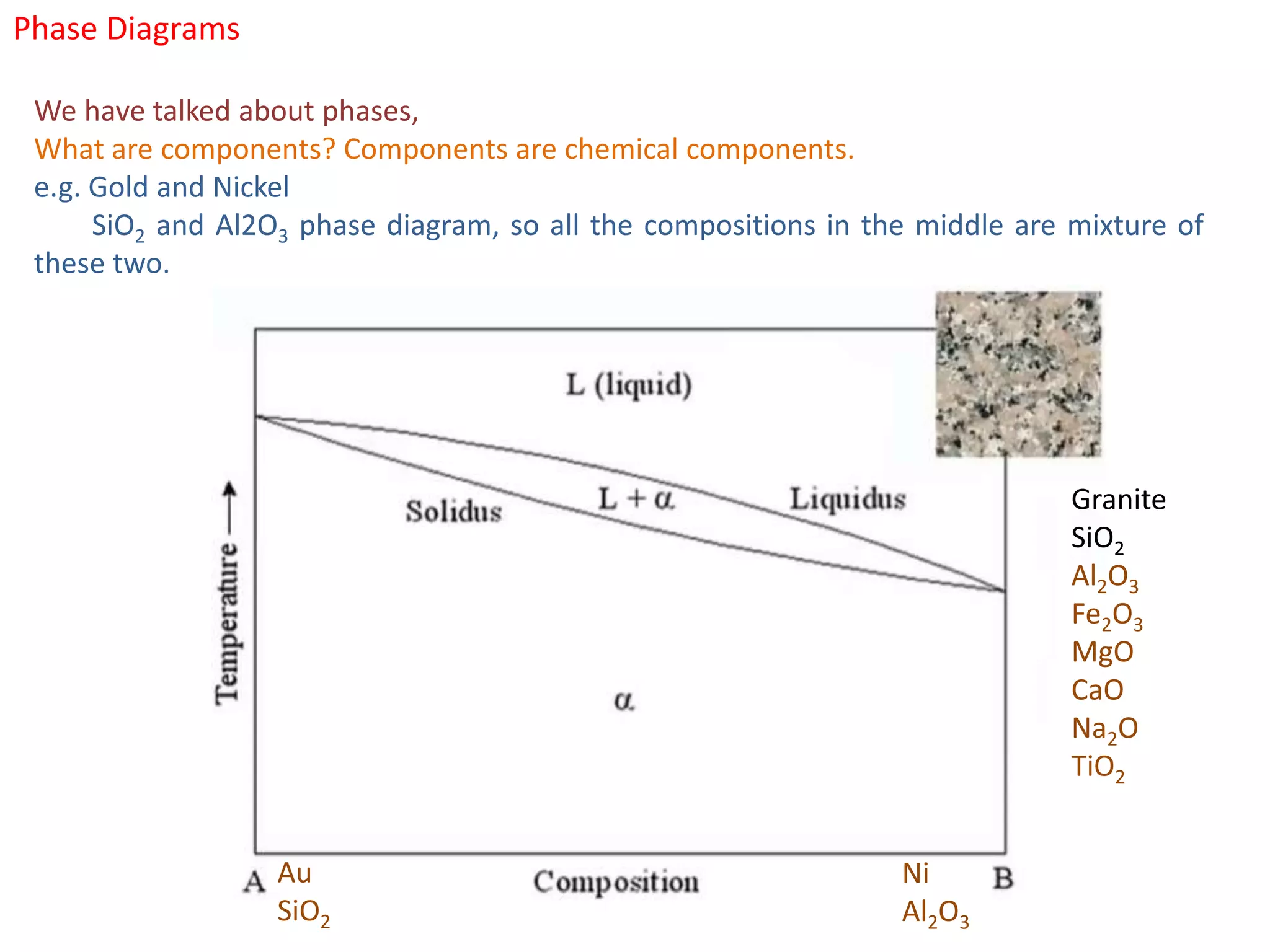
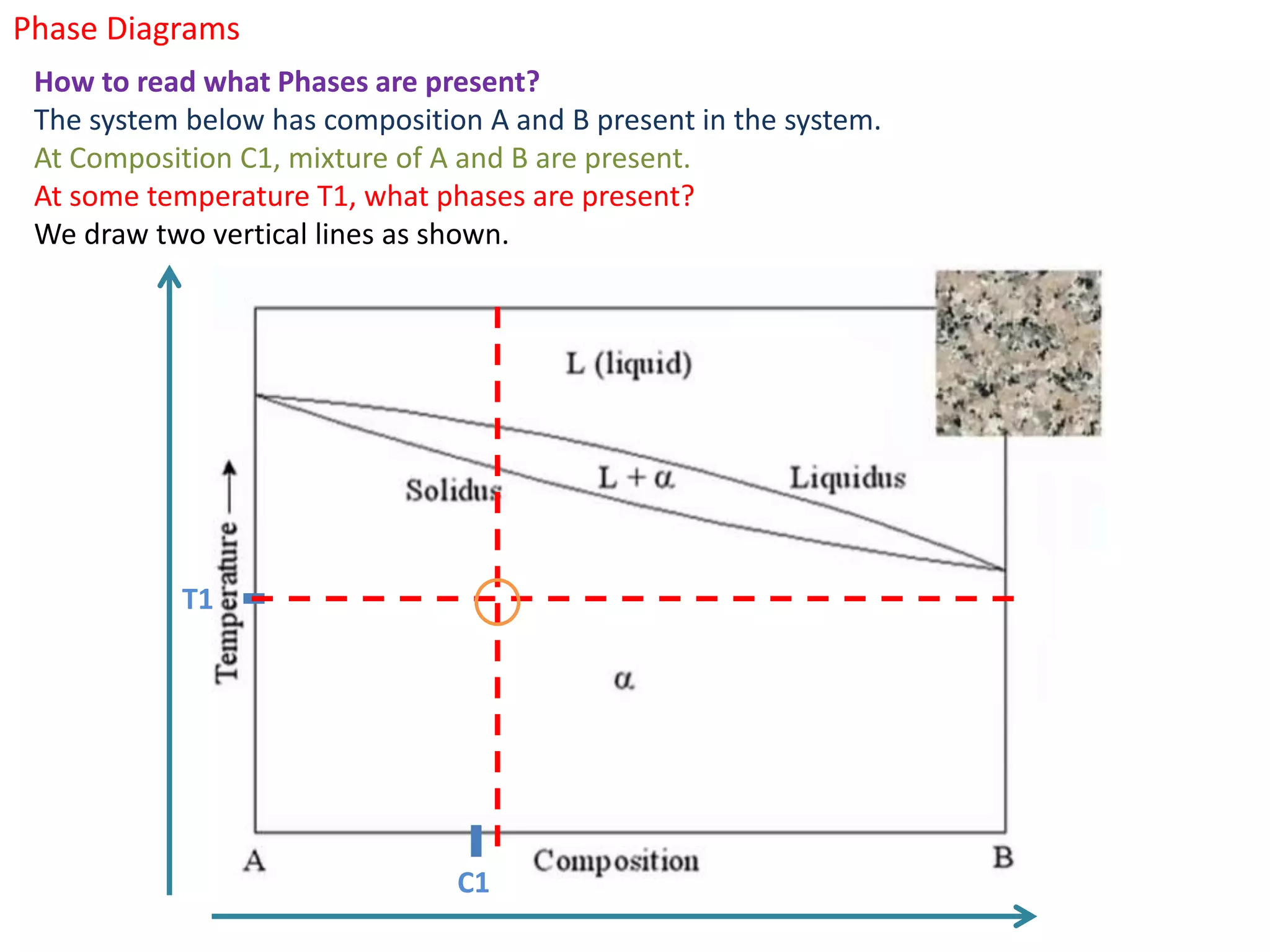
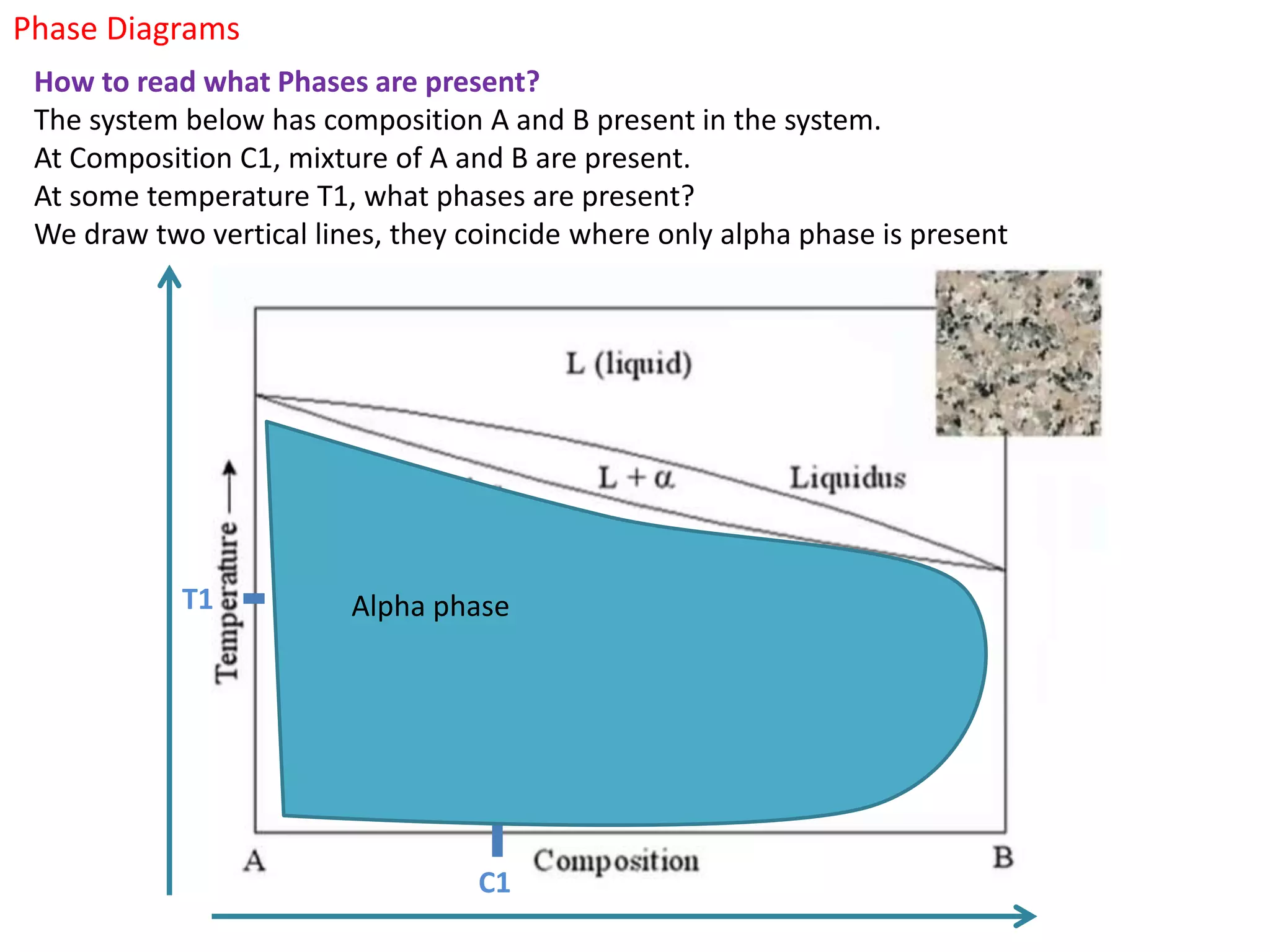
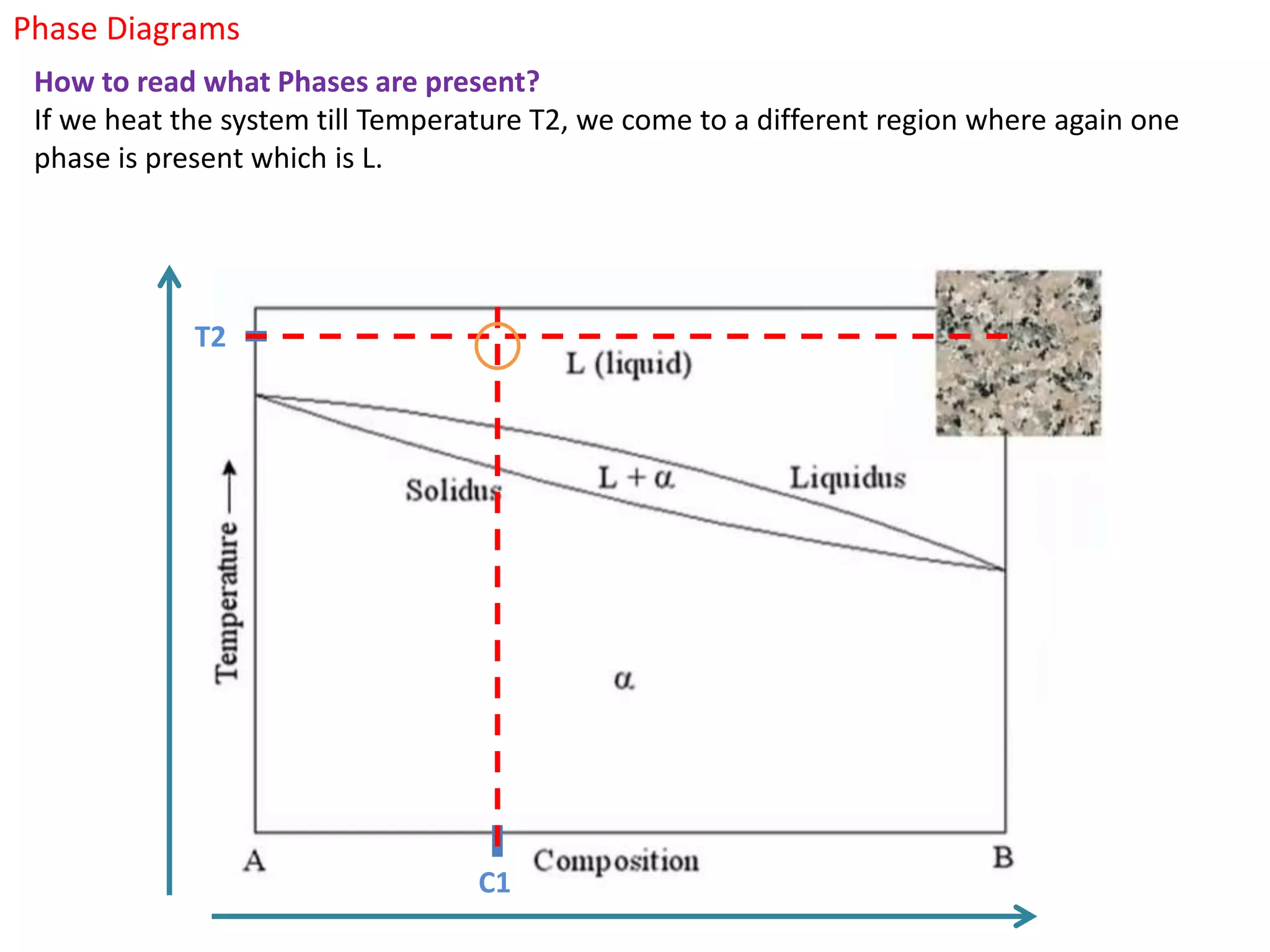
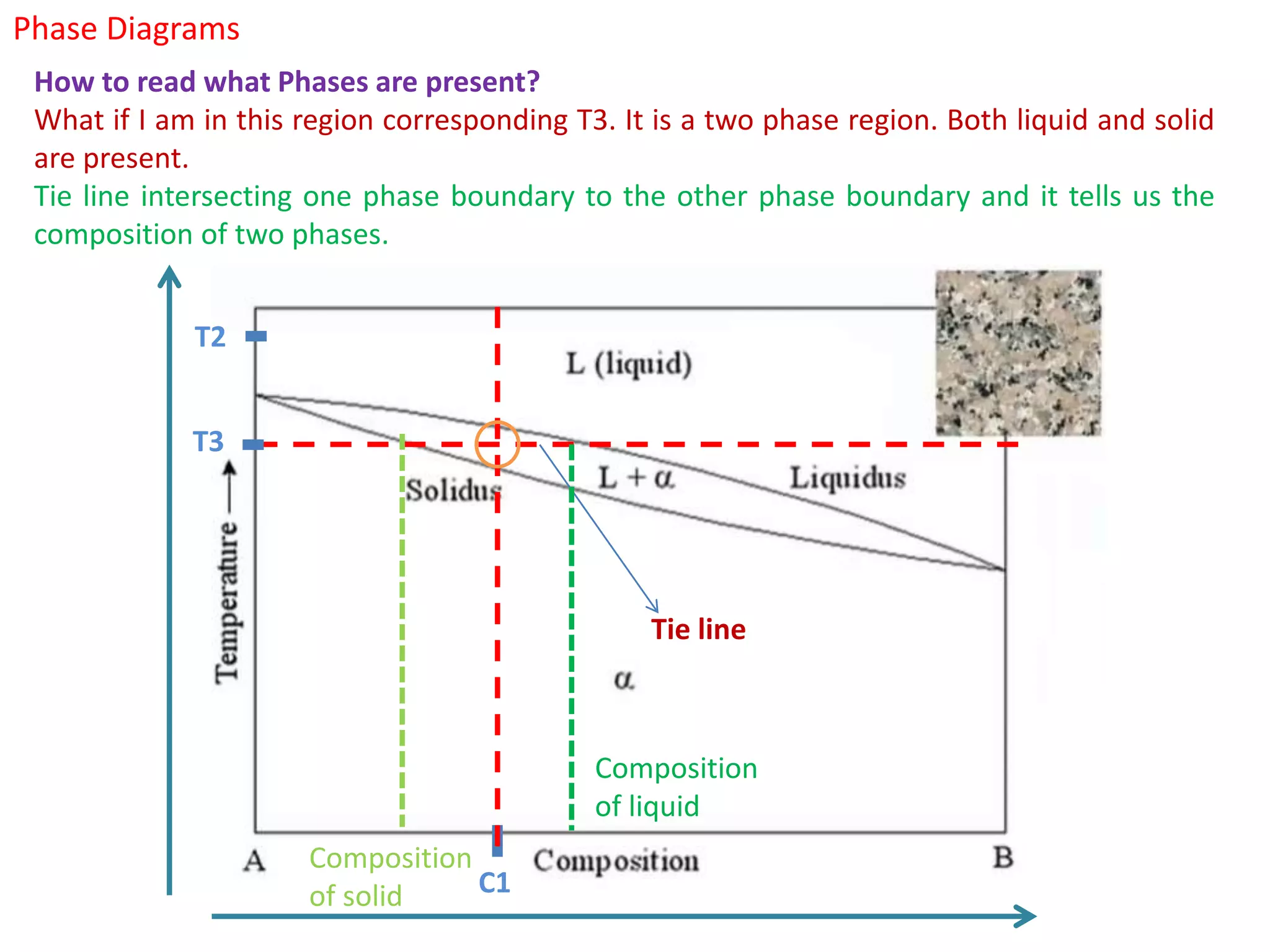
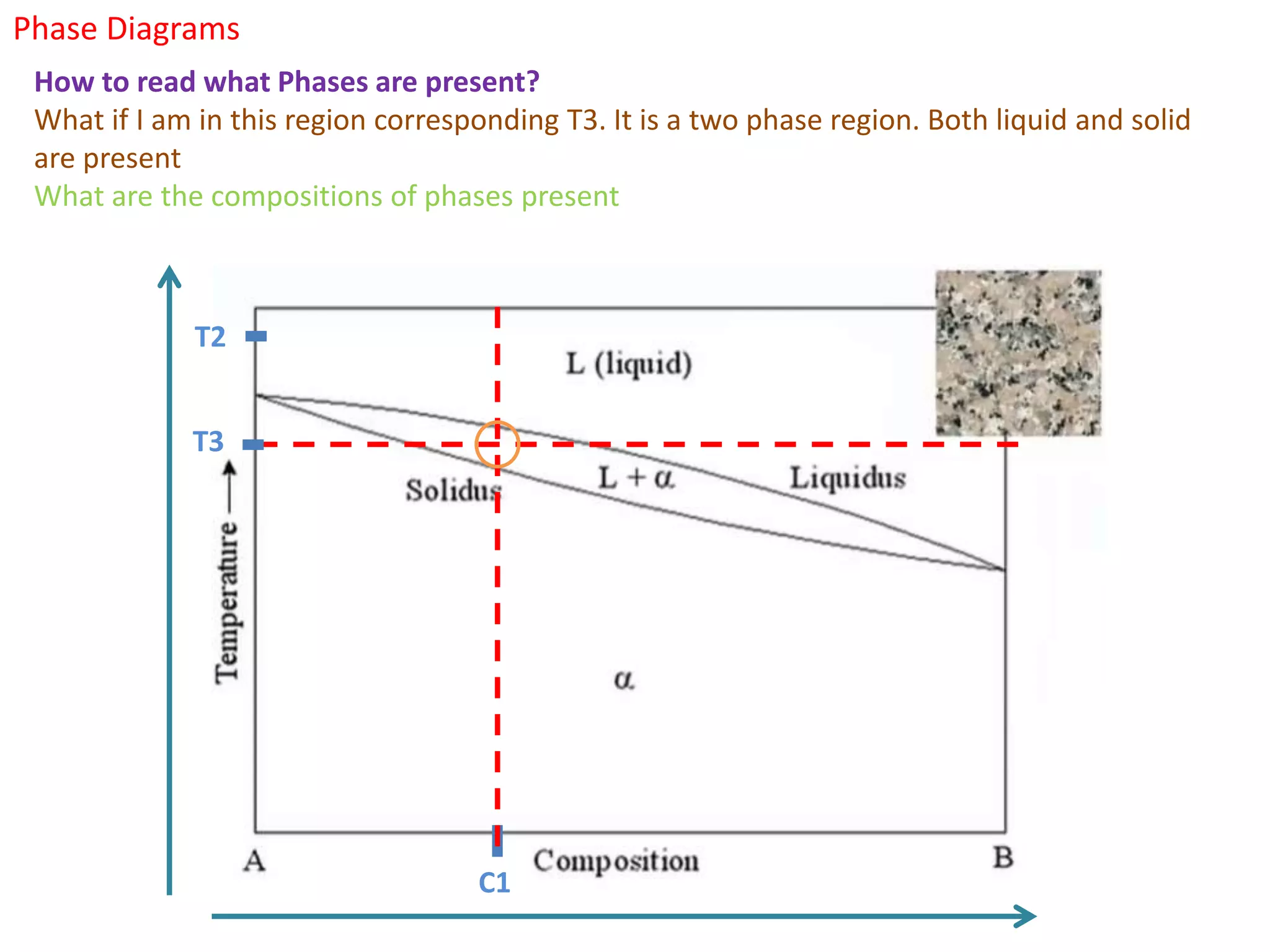
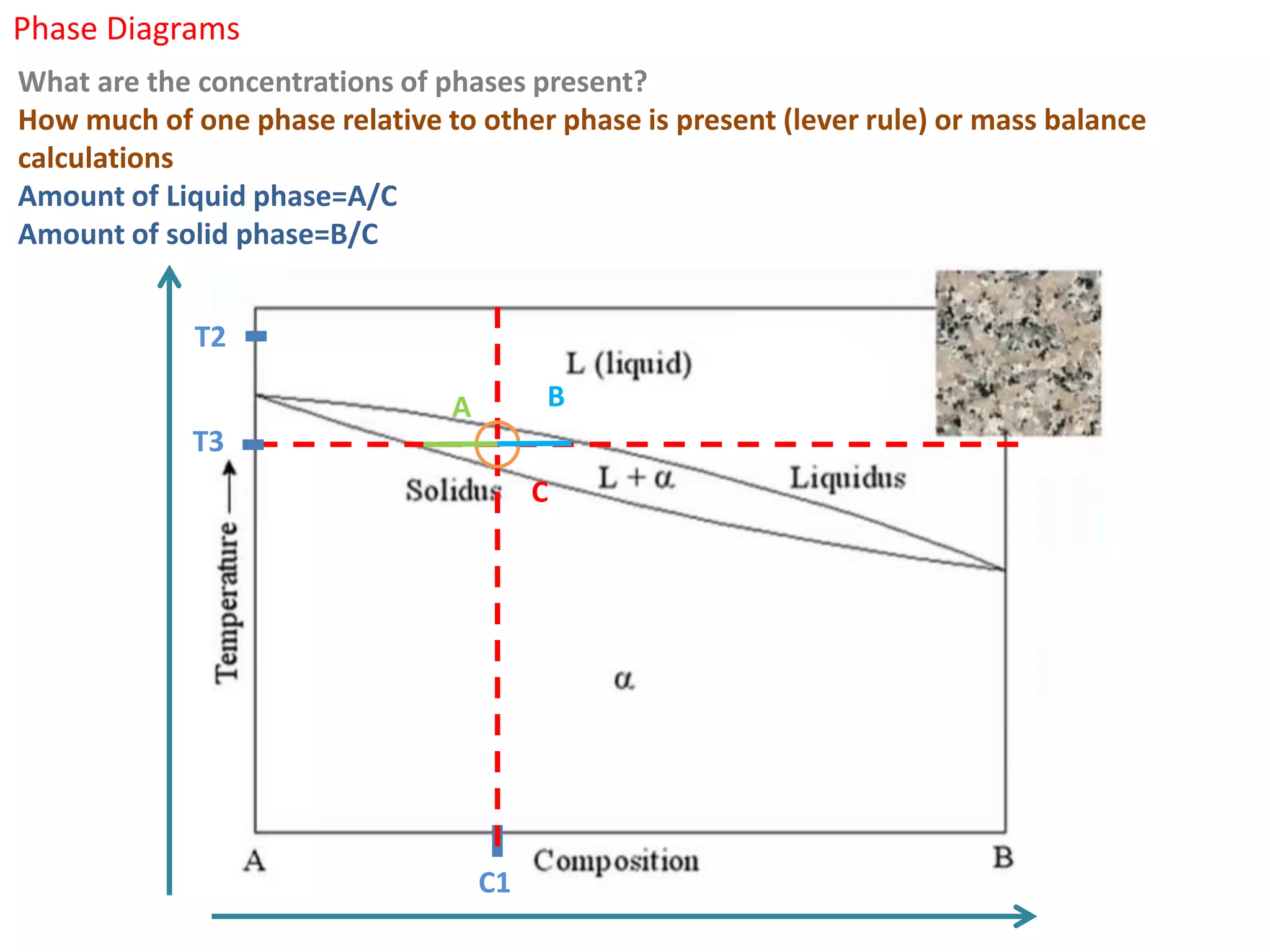
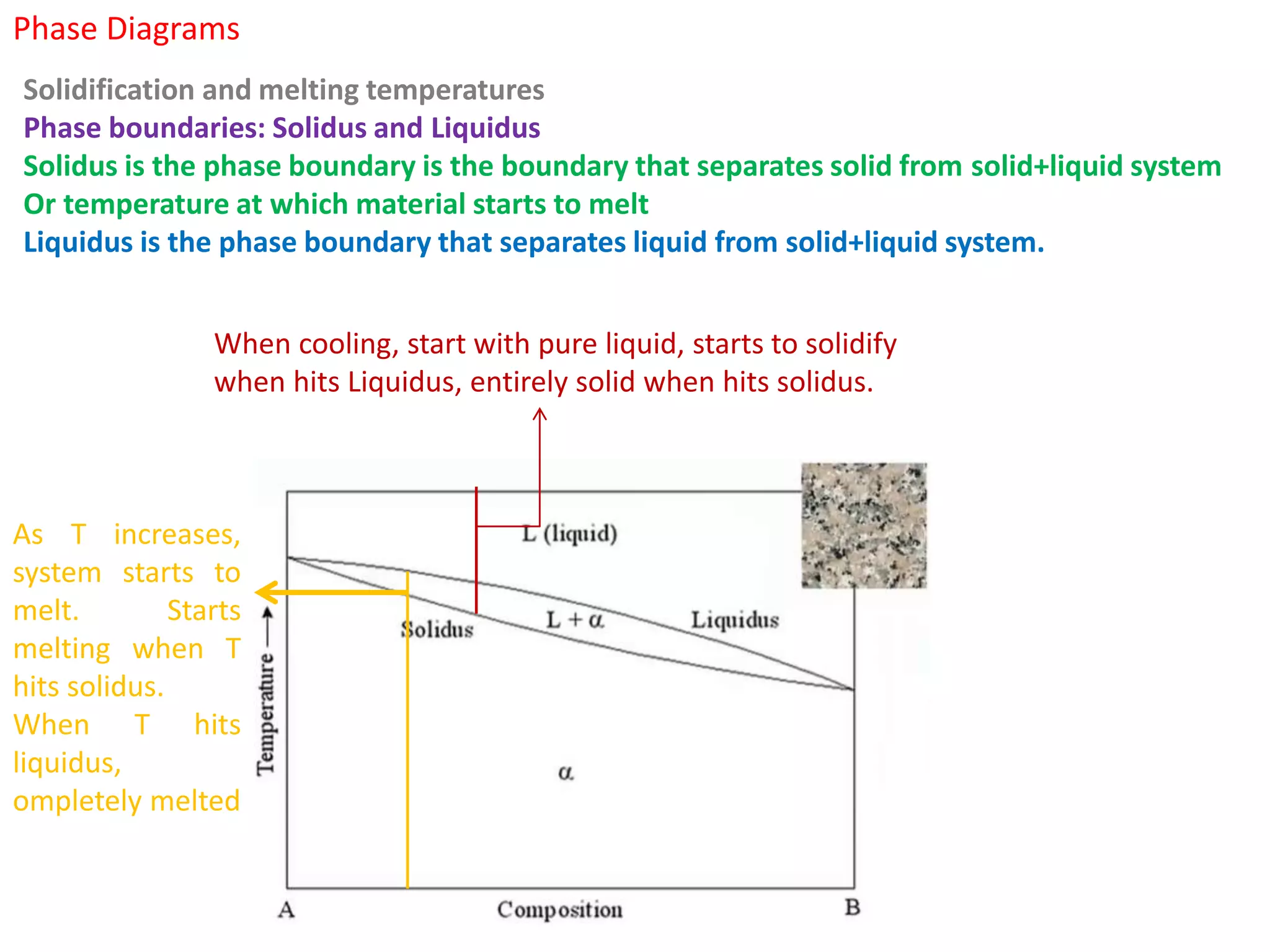
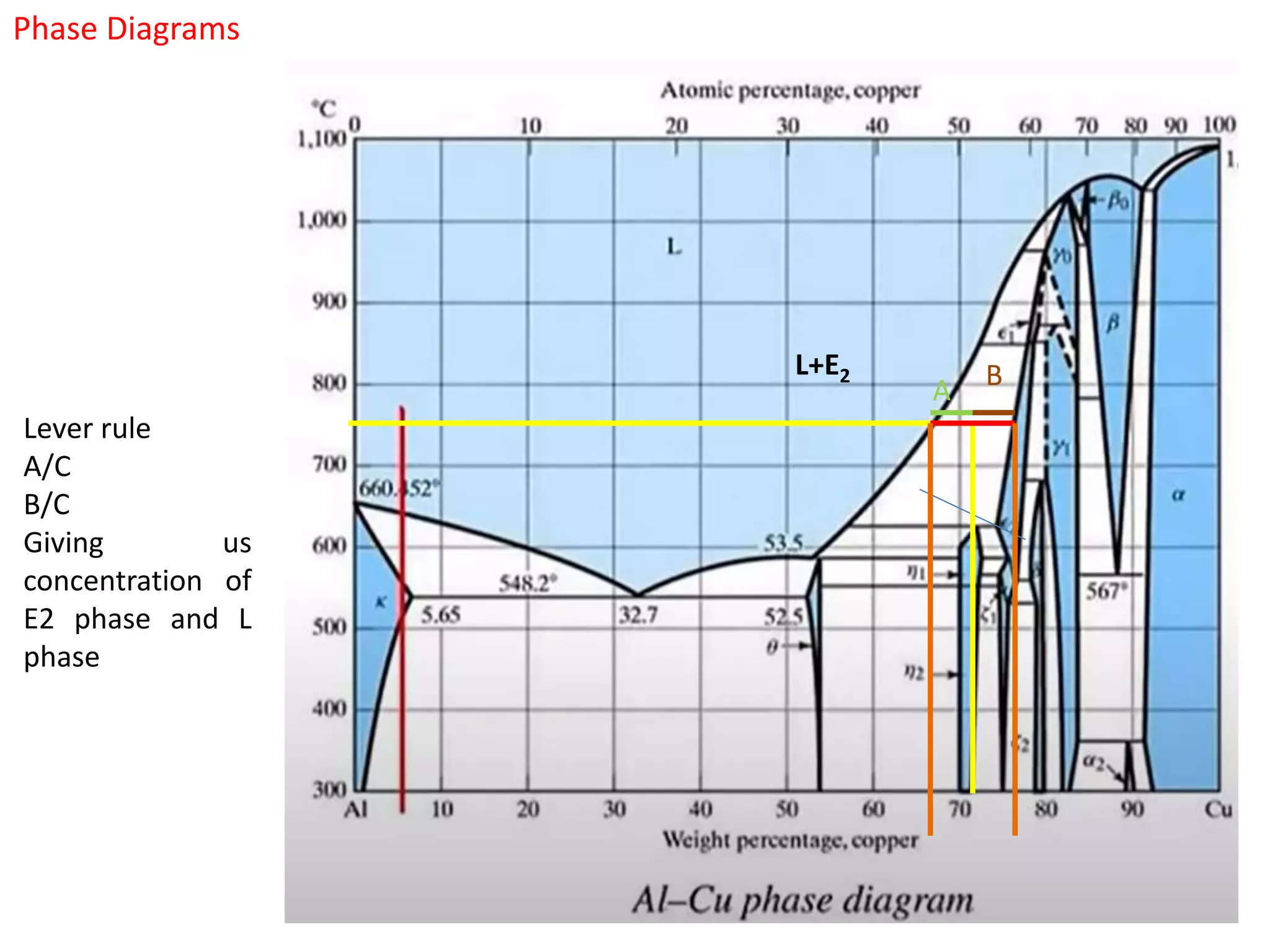
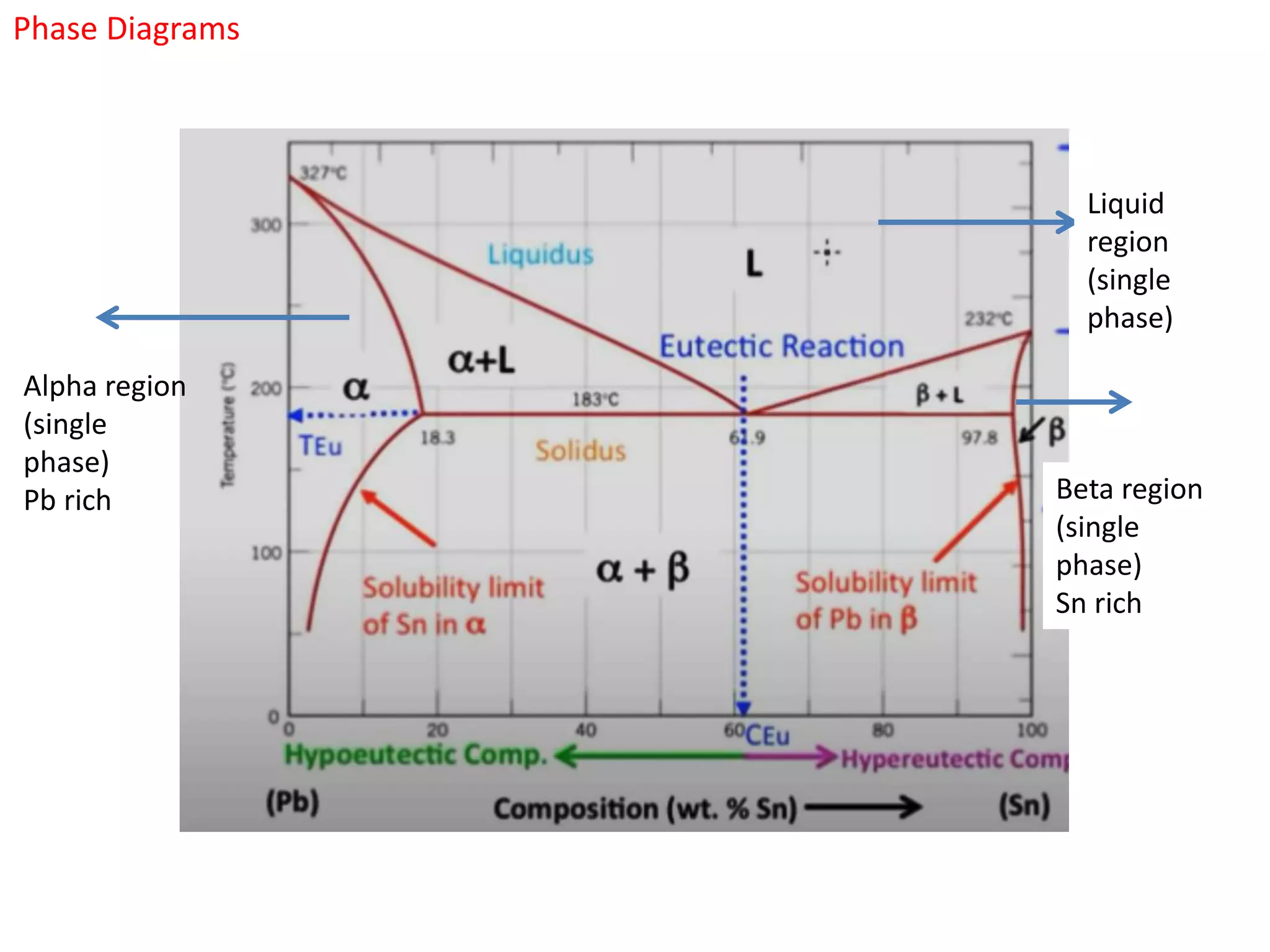
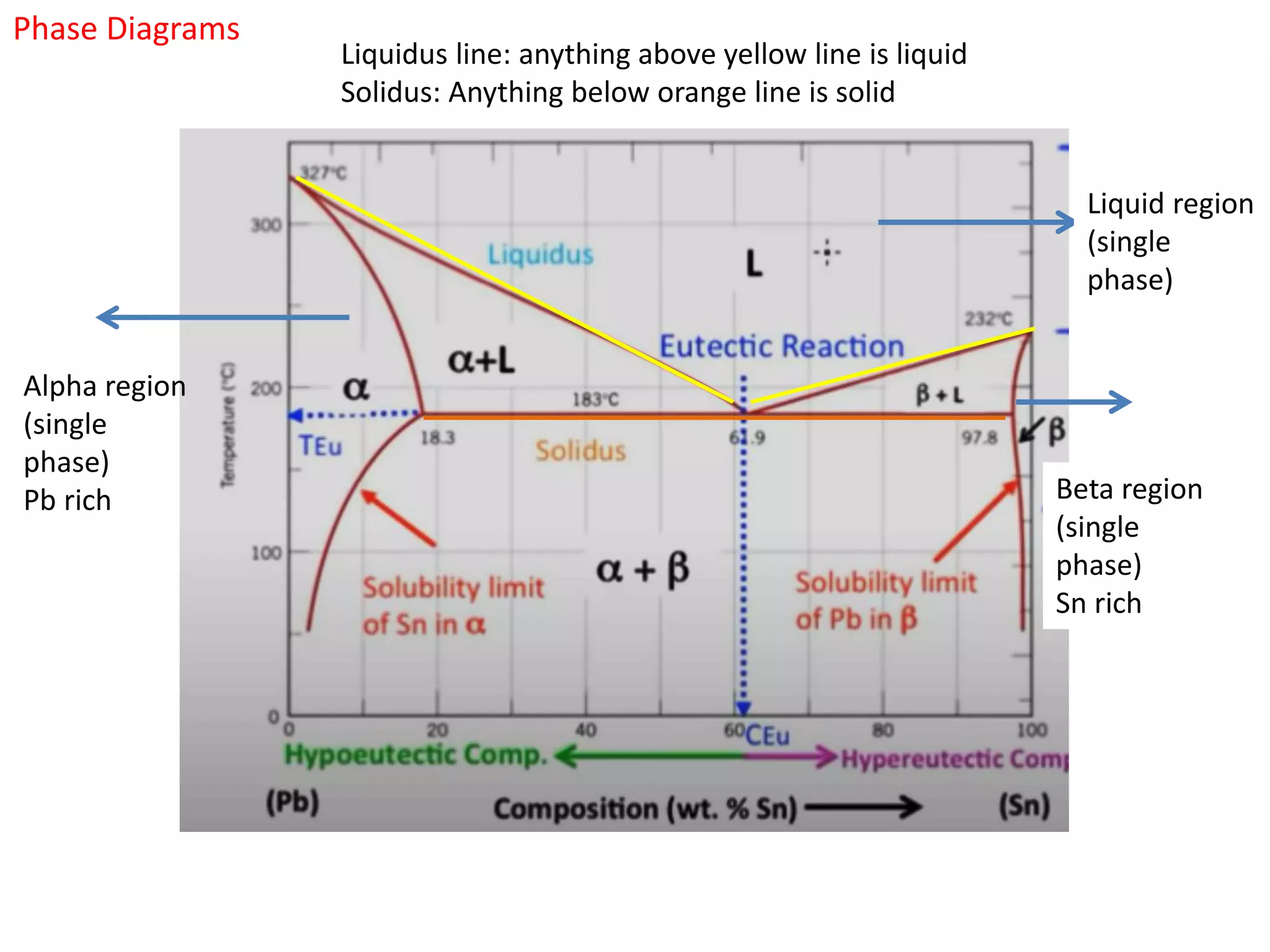
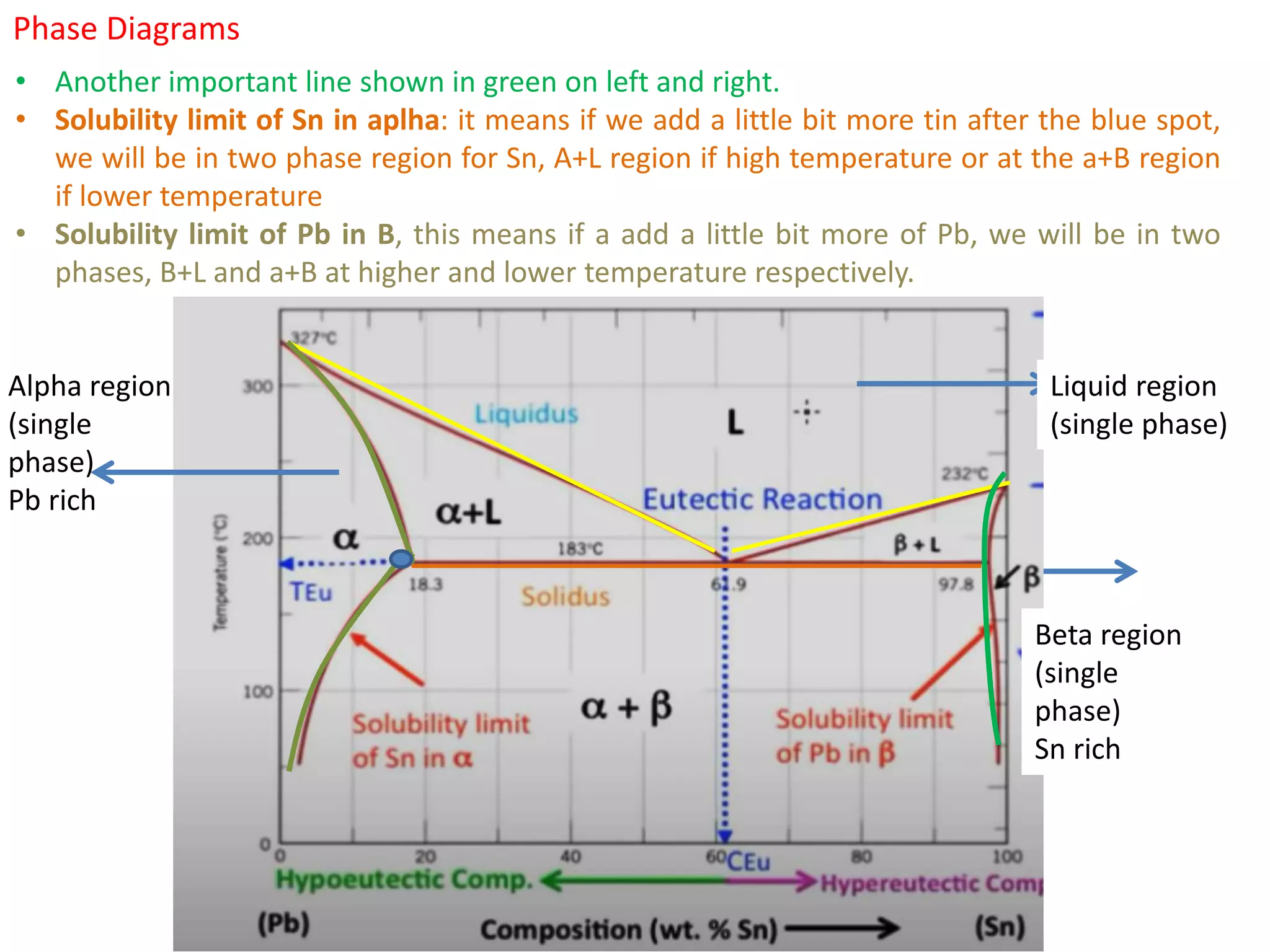
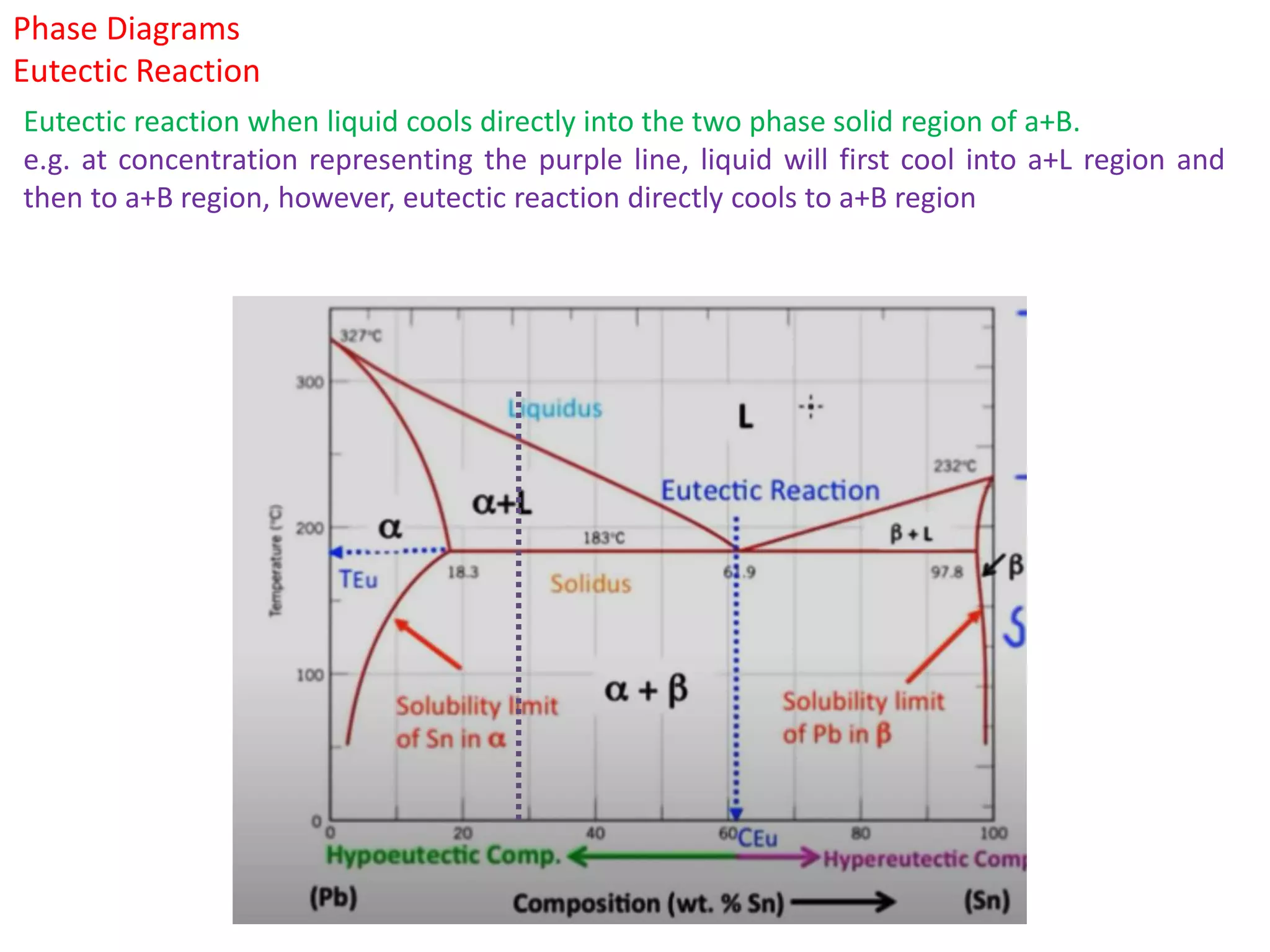
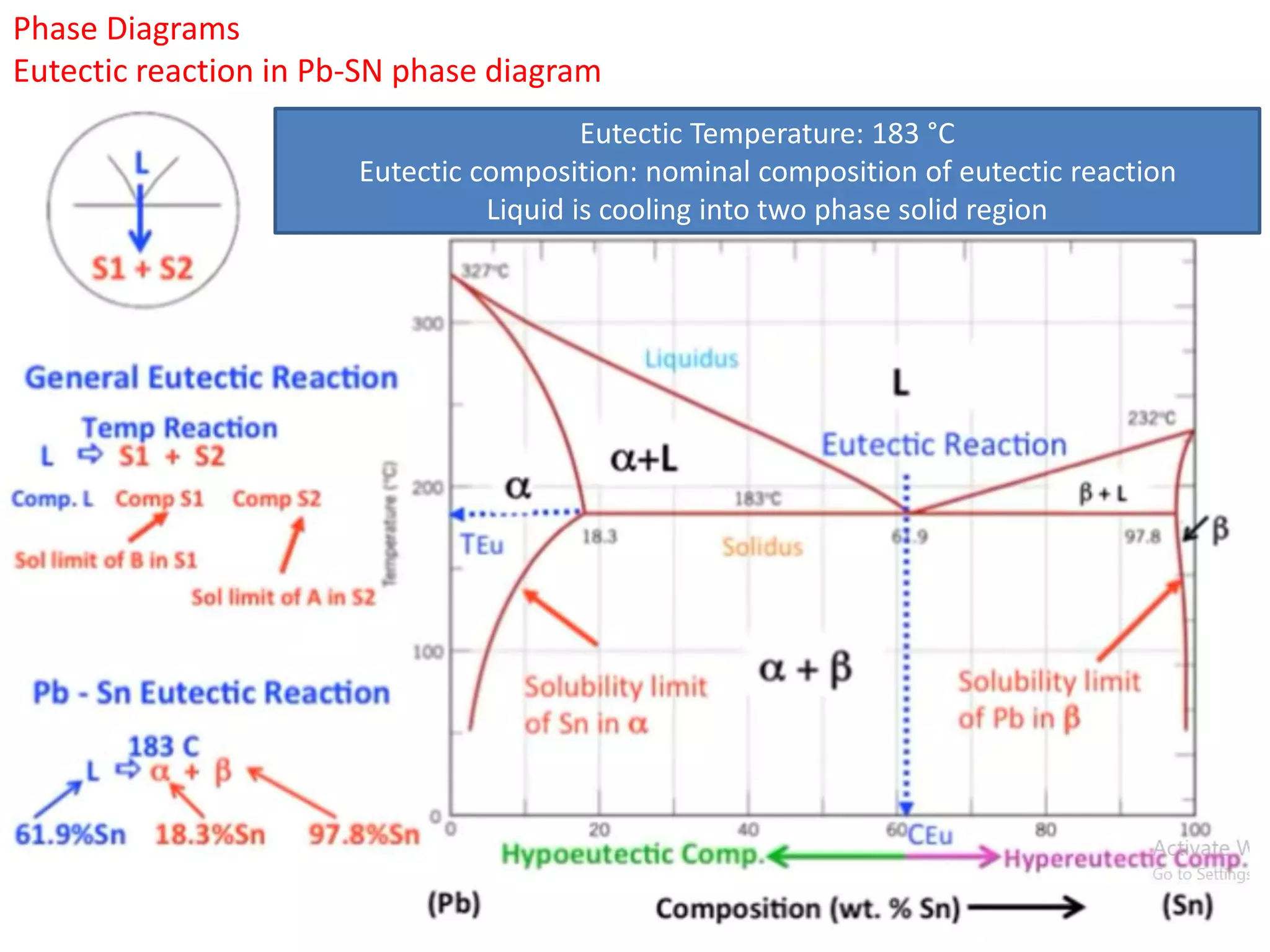
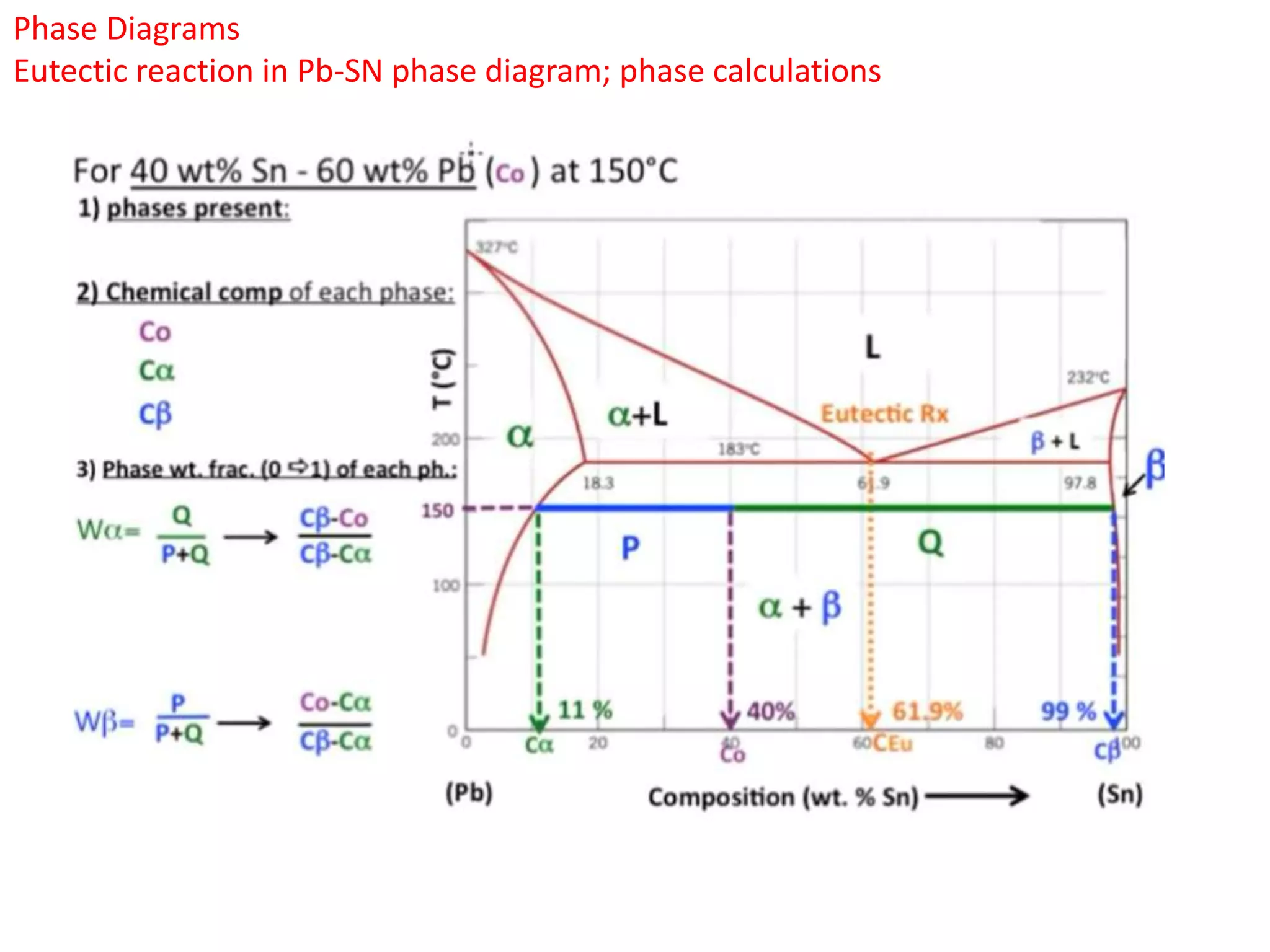
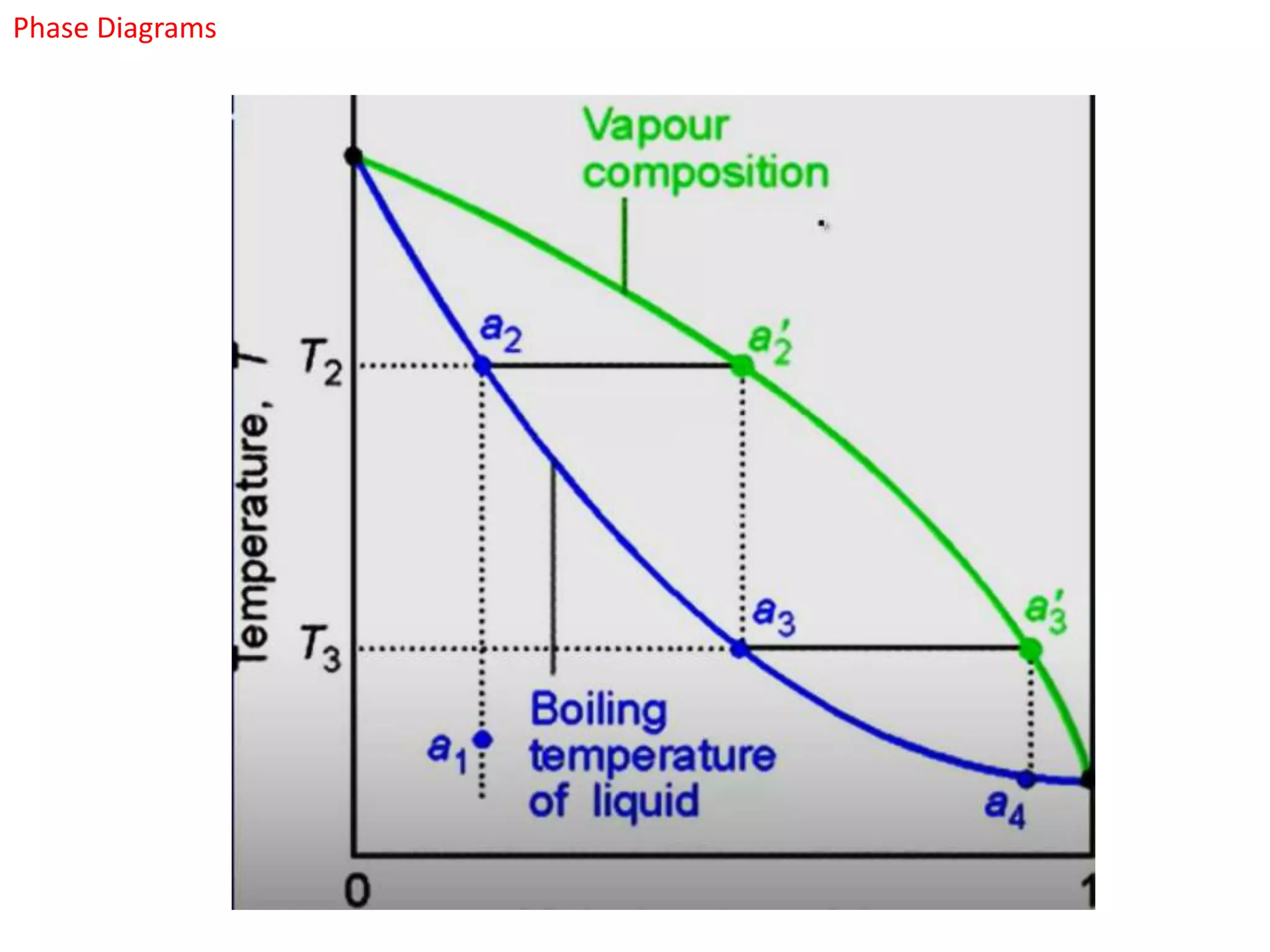
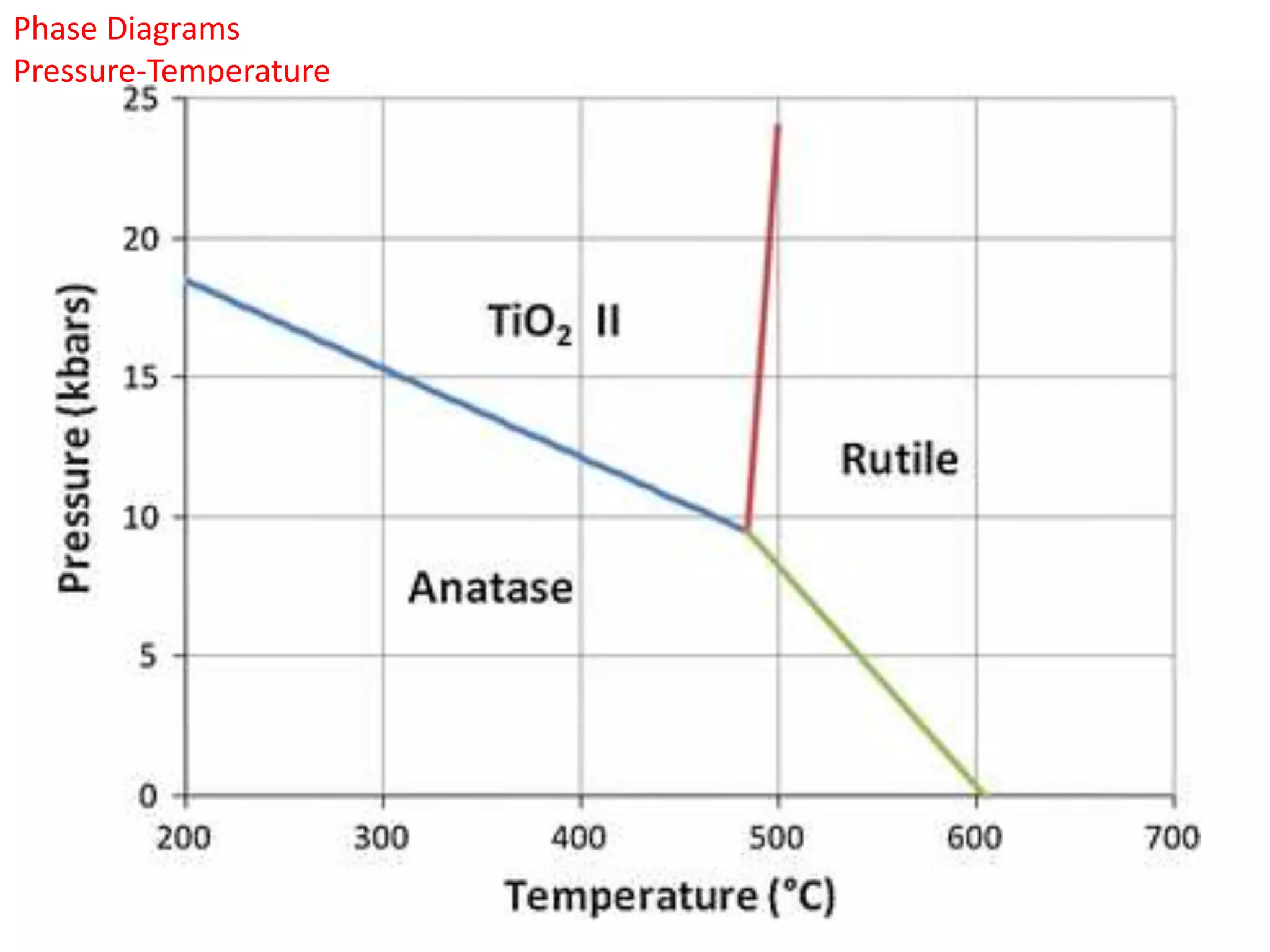
The document provides an overview of phase diagrams, which graphically represent the physical states of a substance under varying temperature and pressure conditions. It discusses the concepts of phases, components, and phase changes, explaining how to interpret information from phase diagrams to determine present phases and their concentrations. Key terms such as solidus, liquidus, and eutectic reactions are defined, along with examples involving binary phase diagrams.