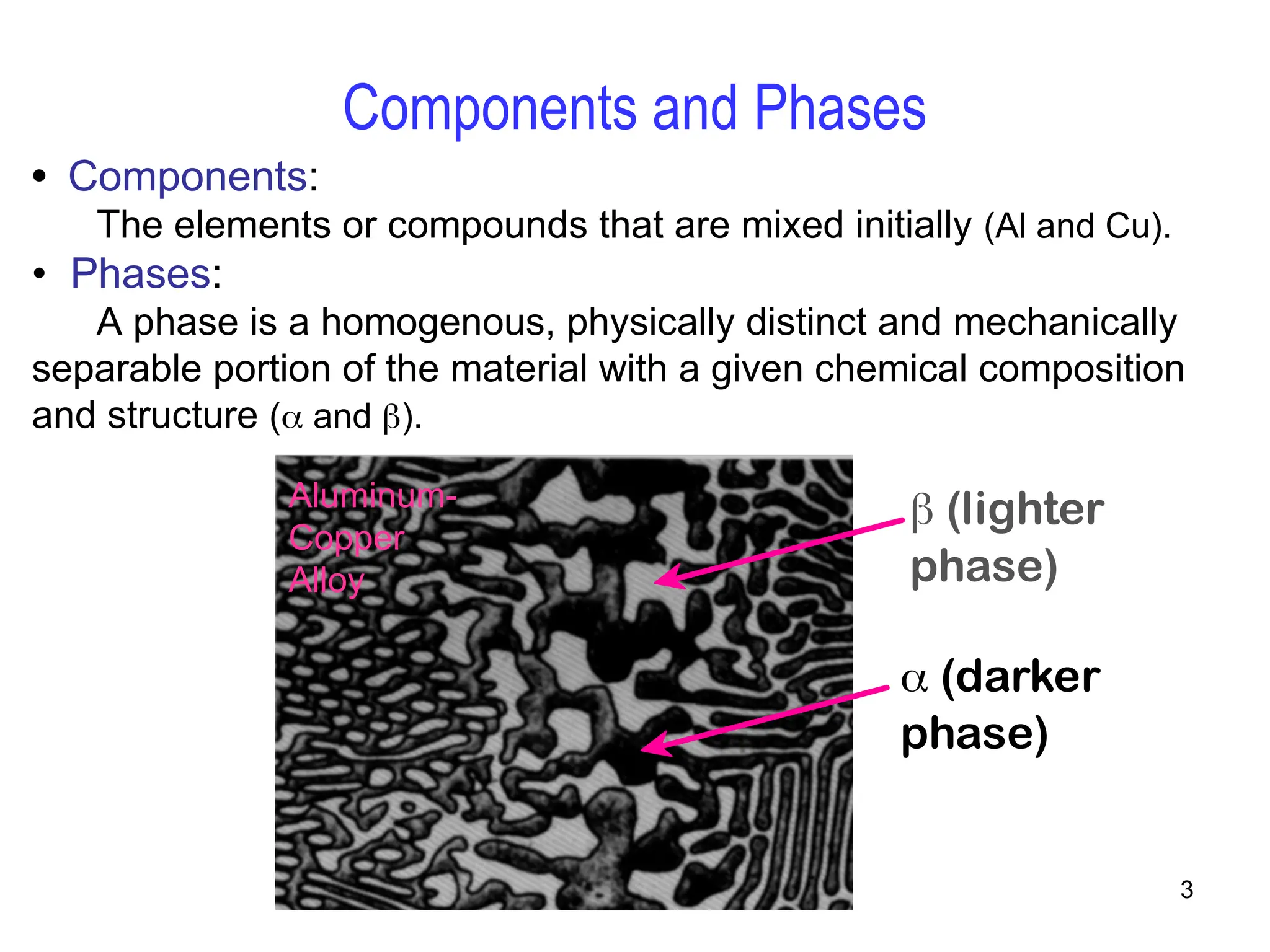
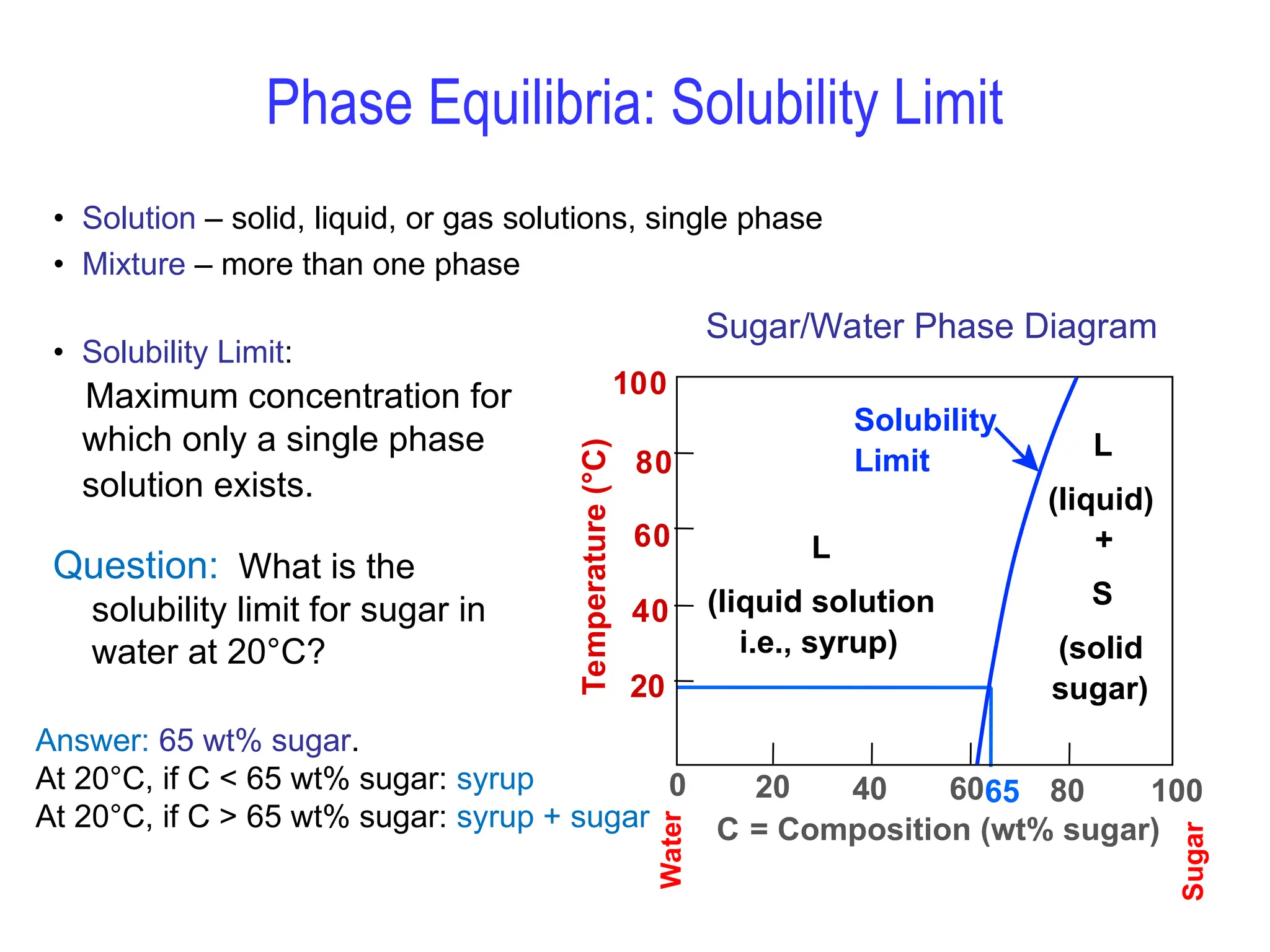
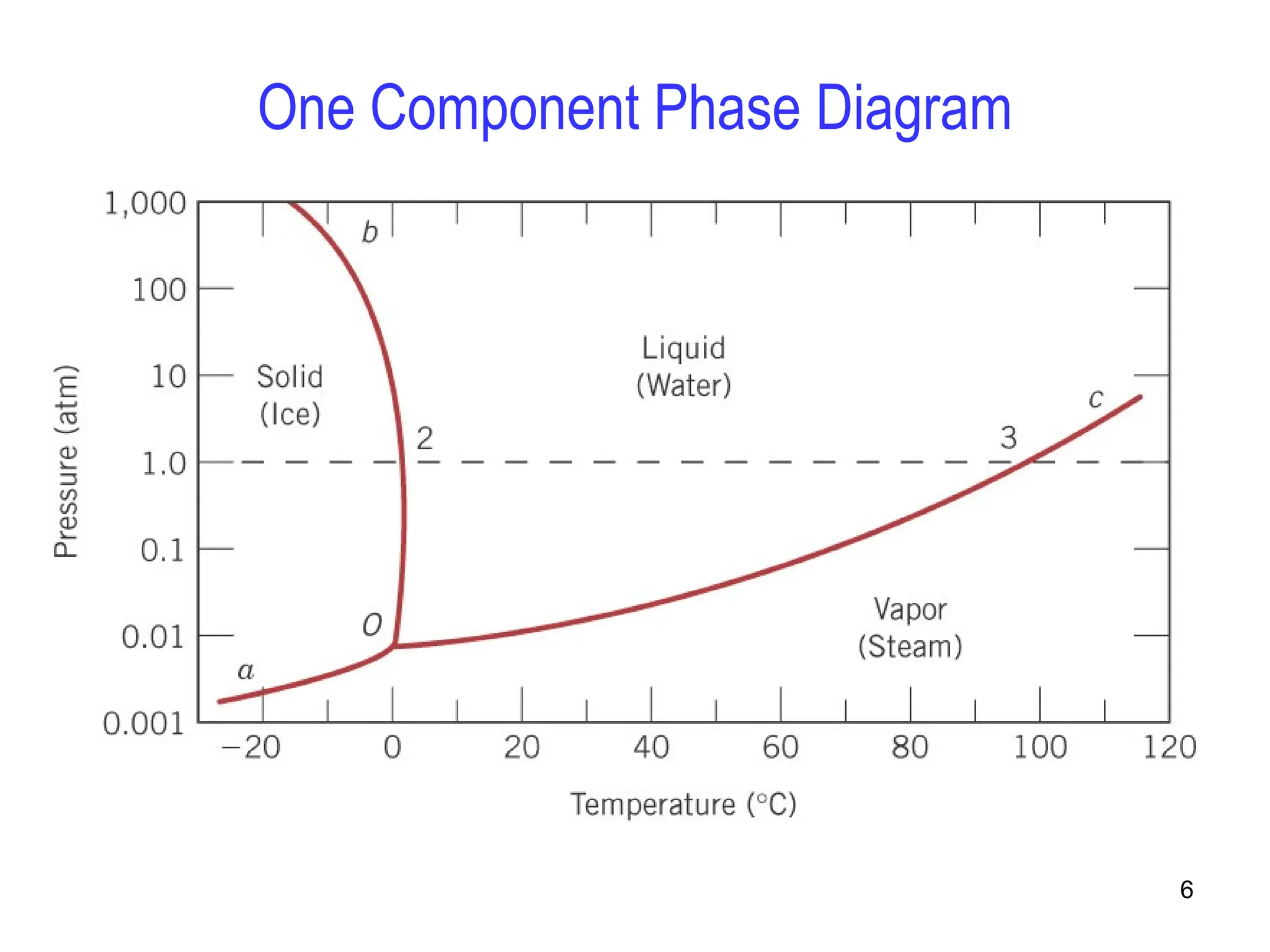
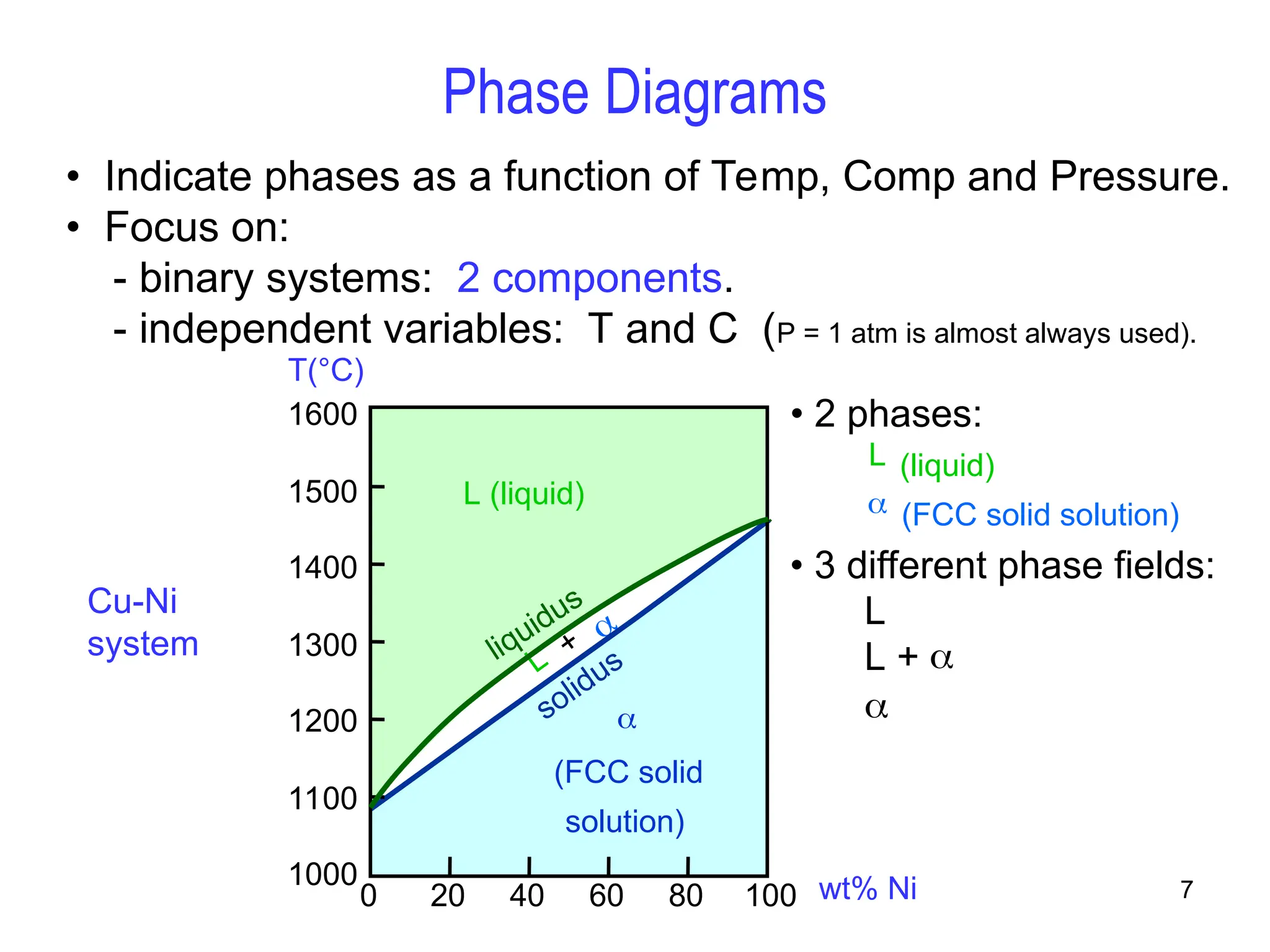
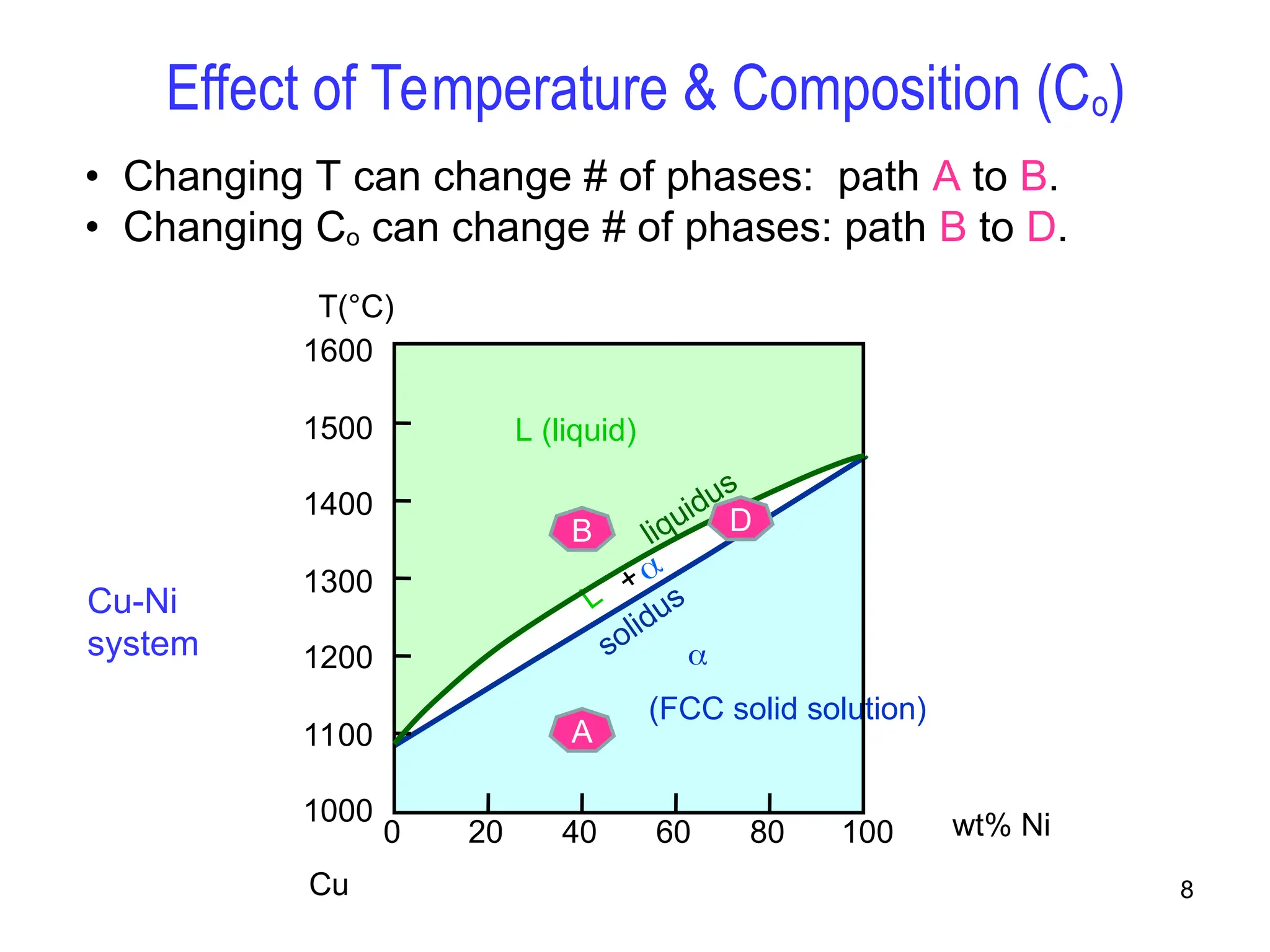
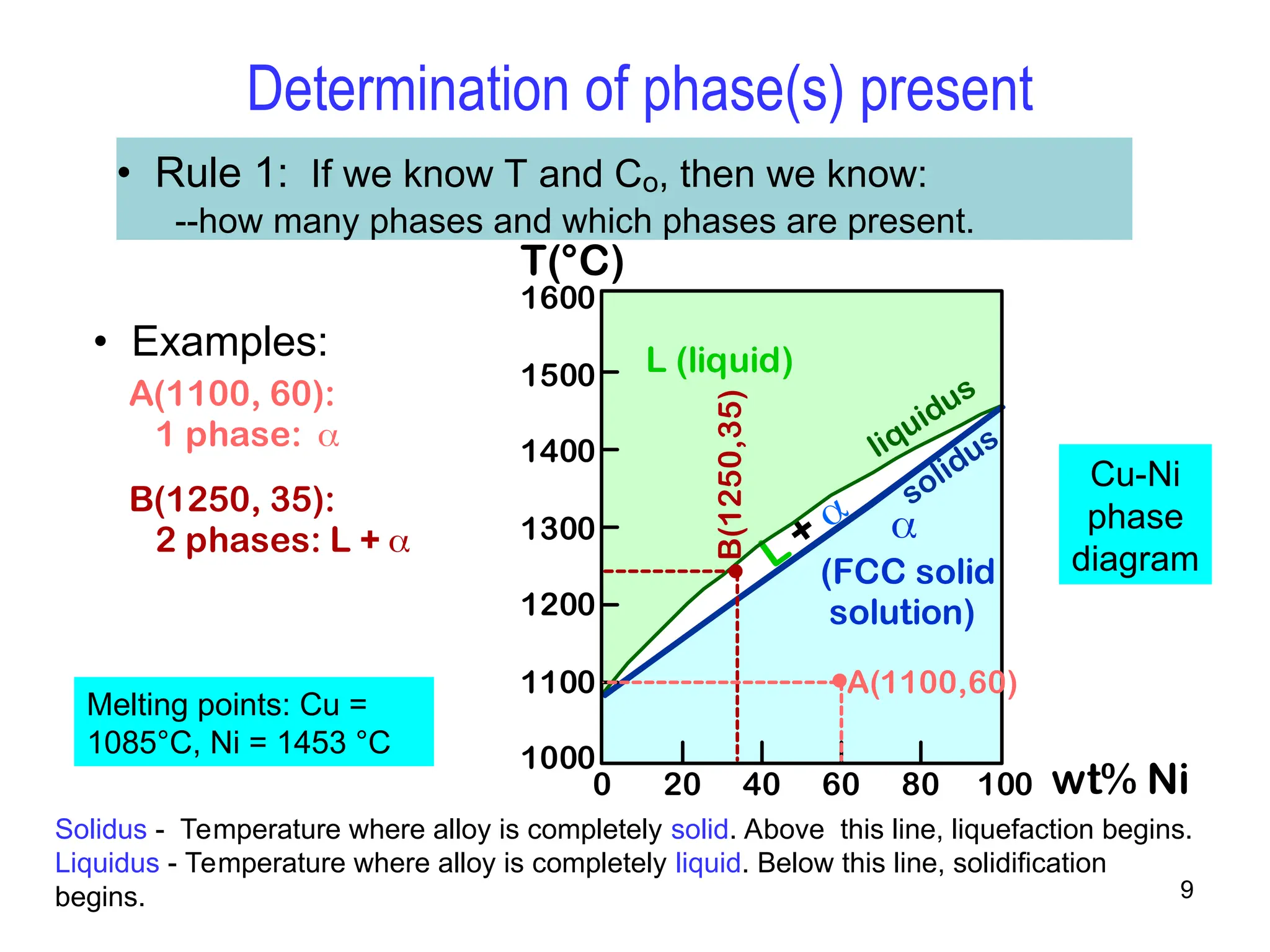
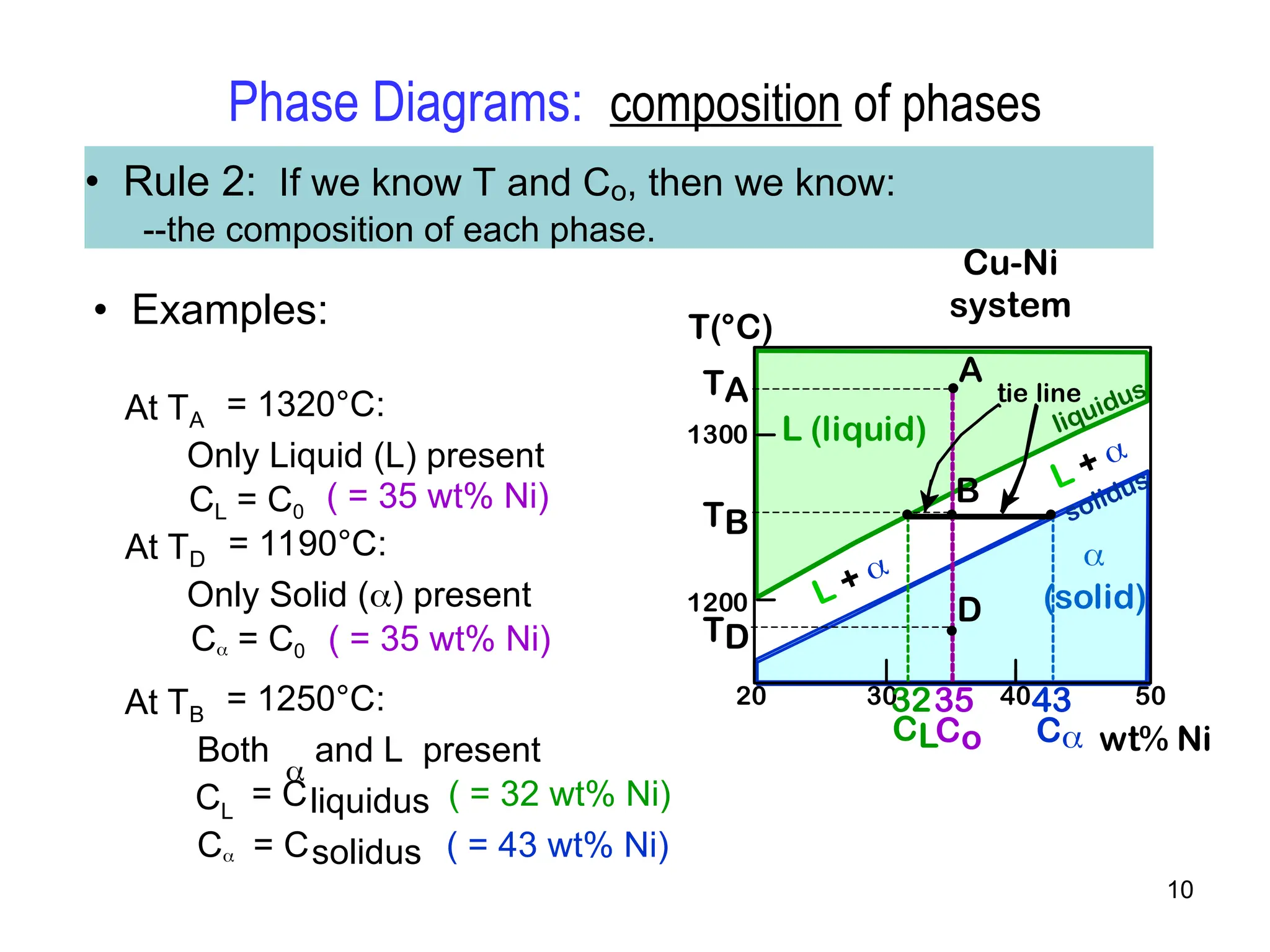
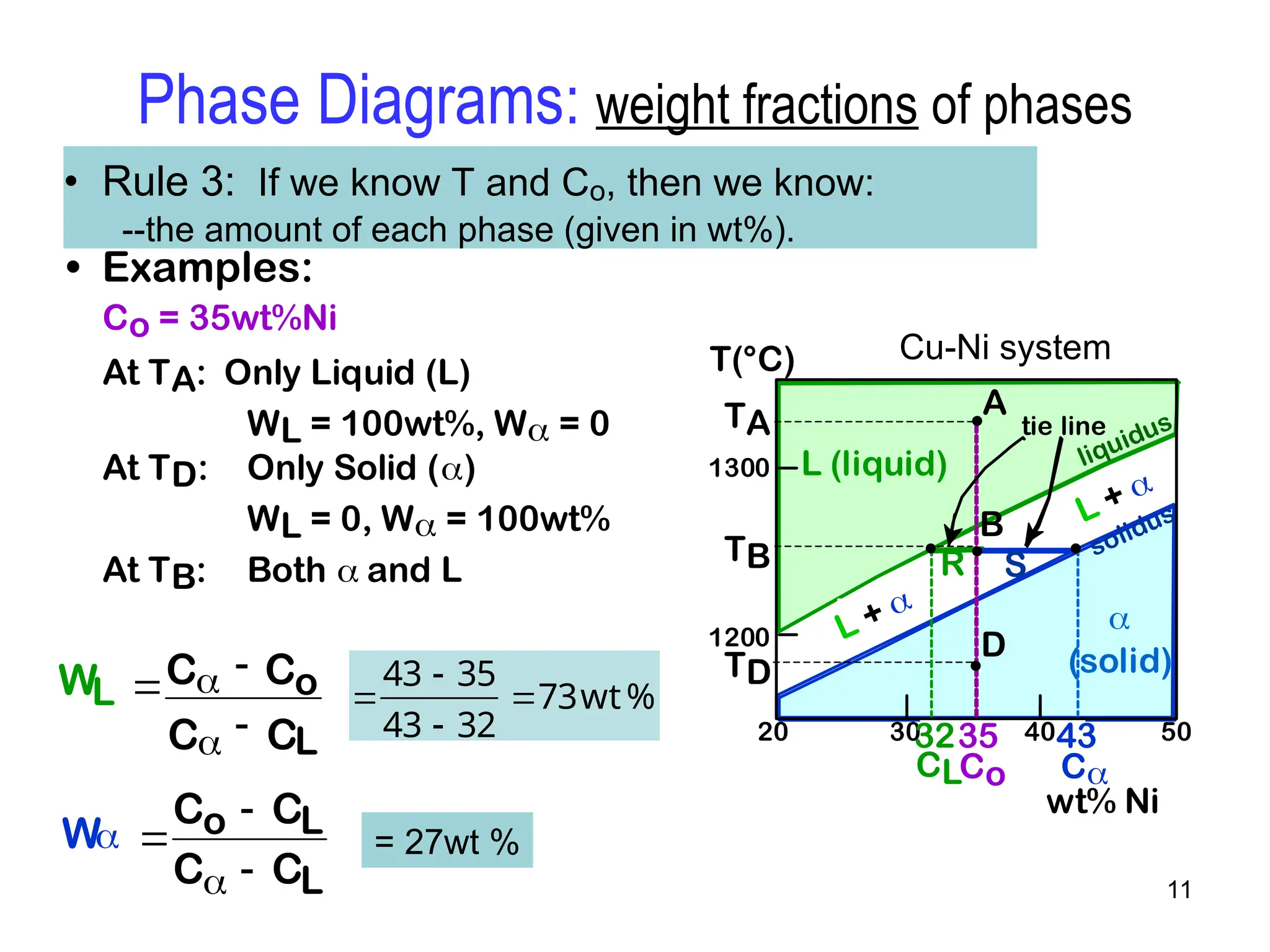
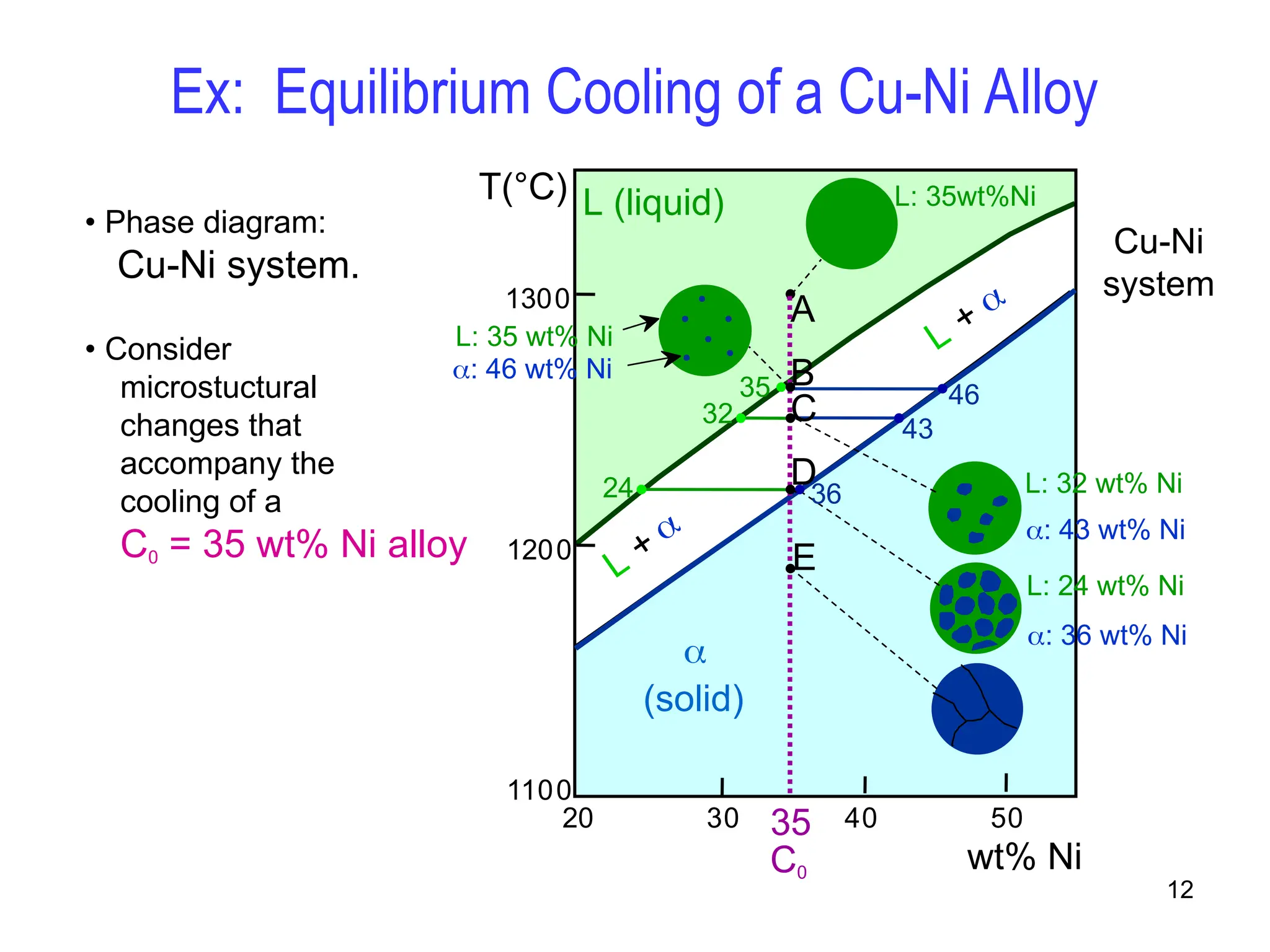
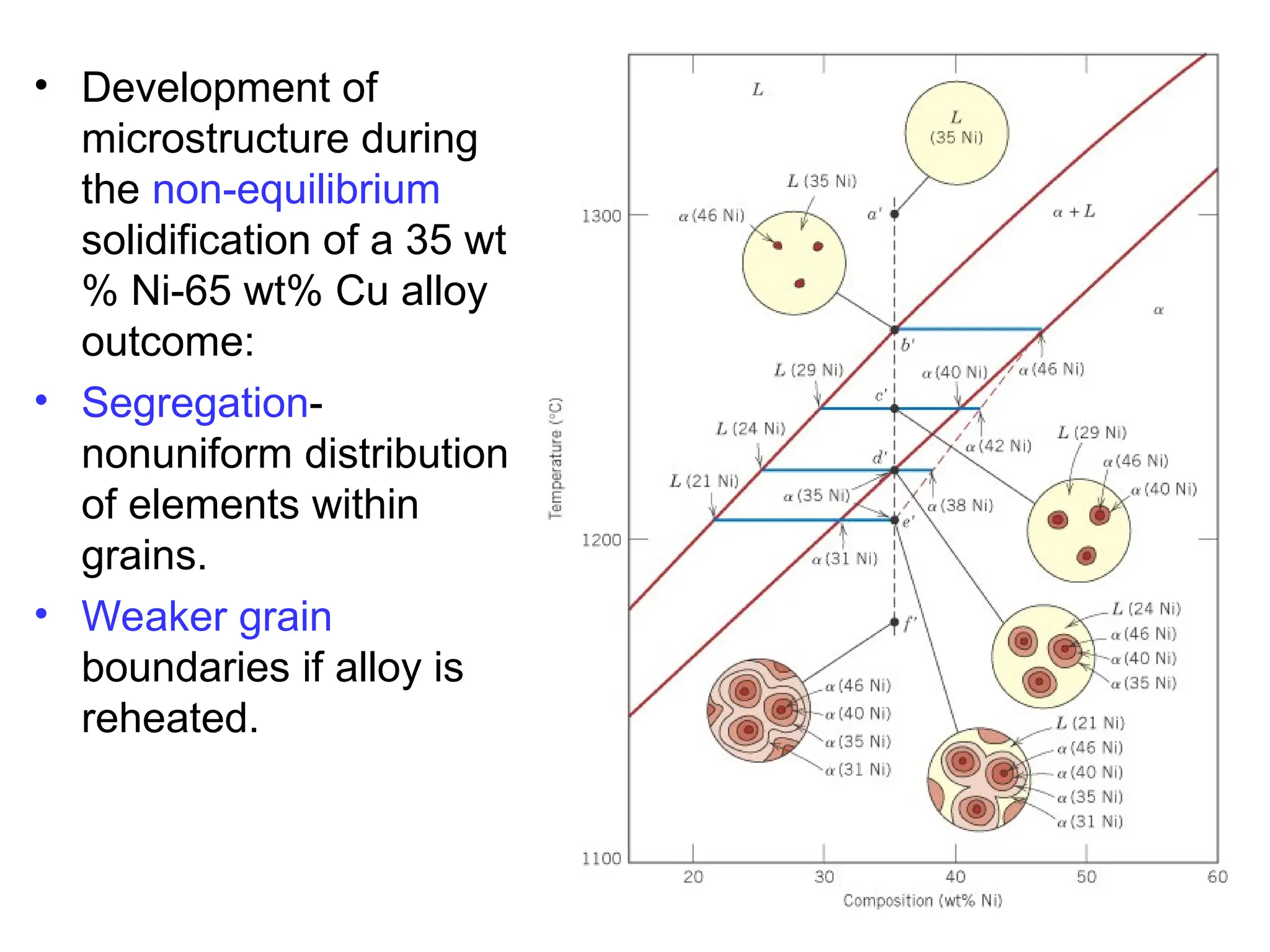
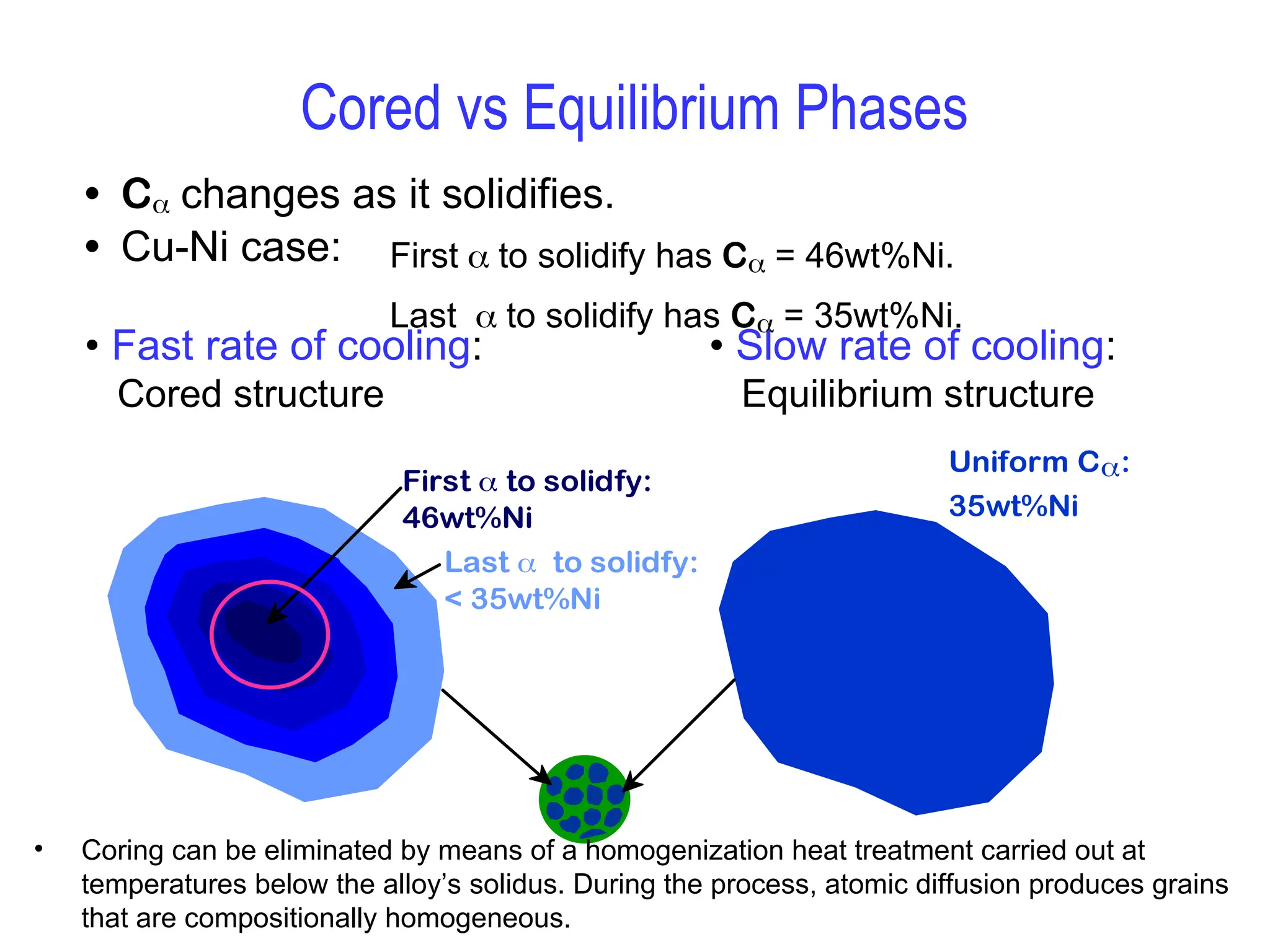
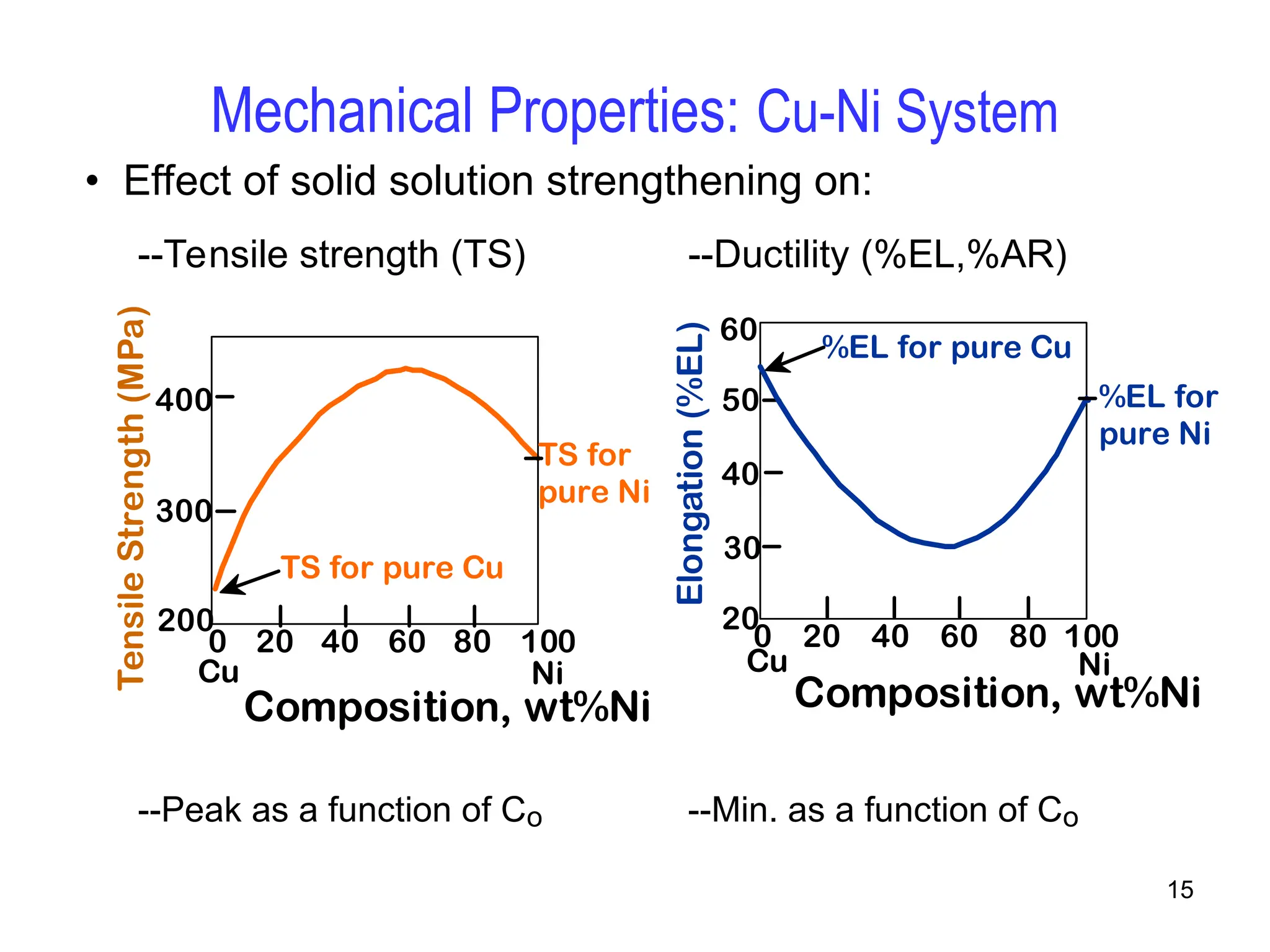
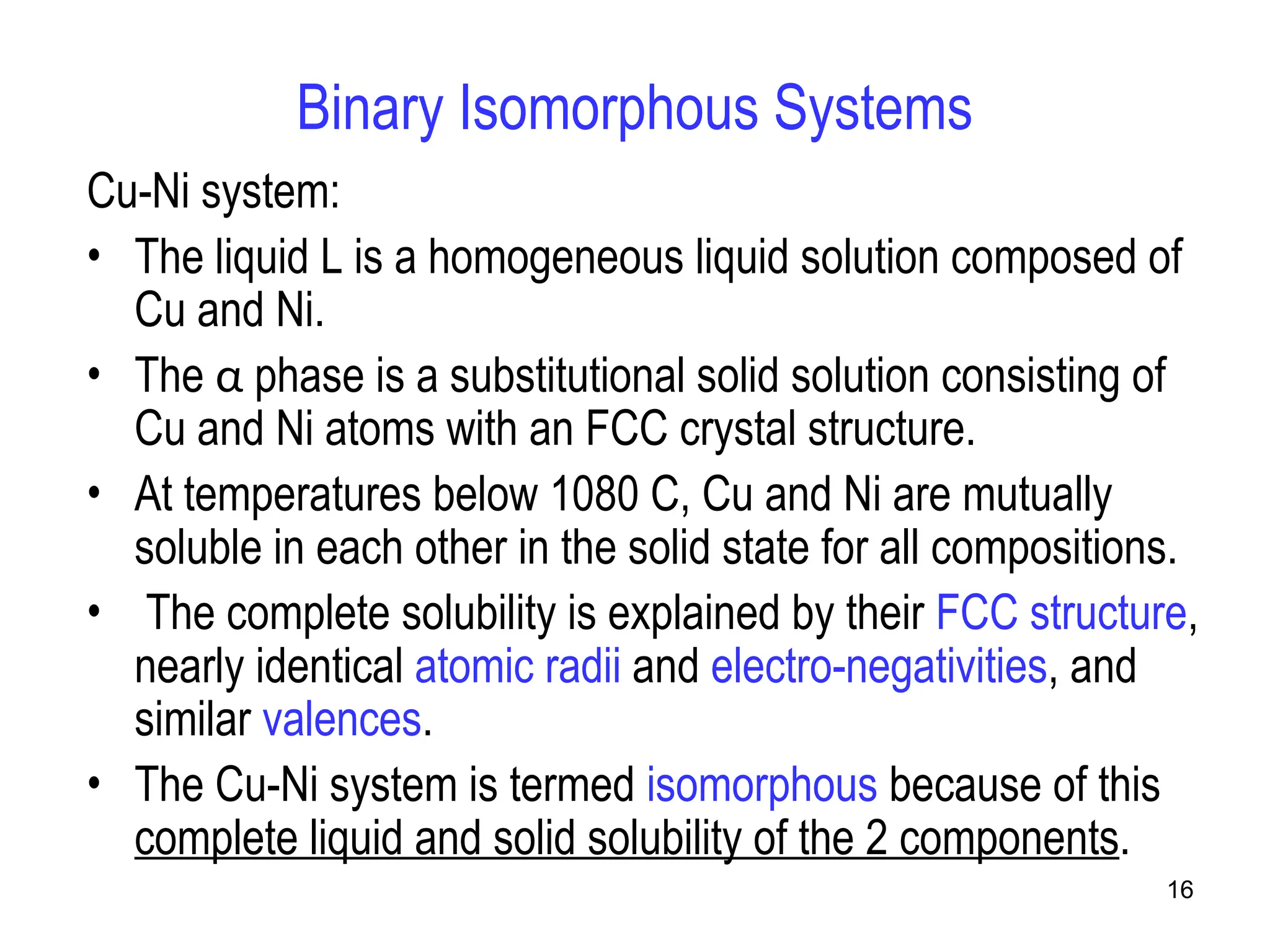
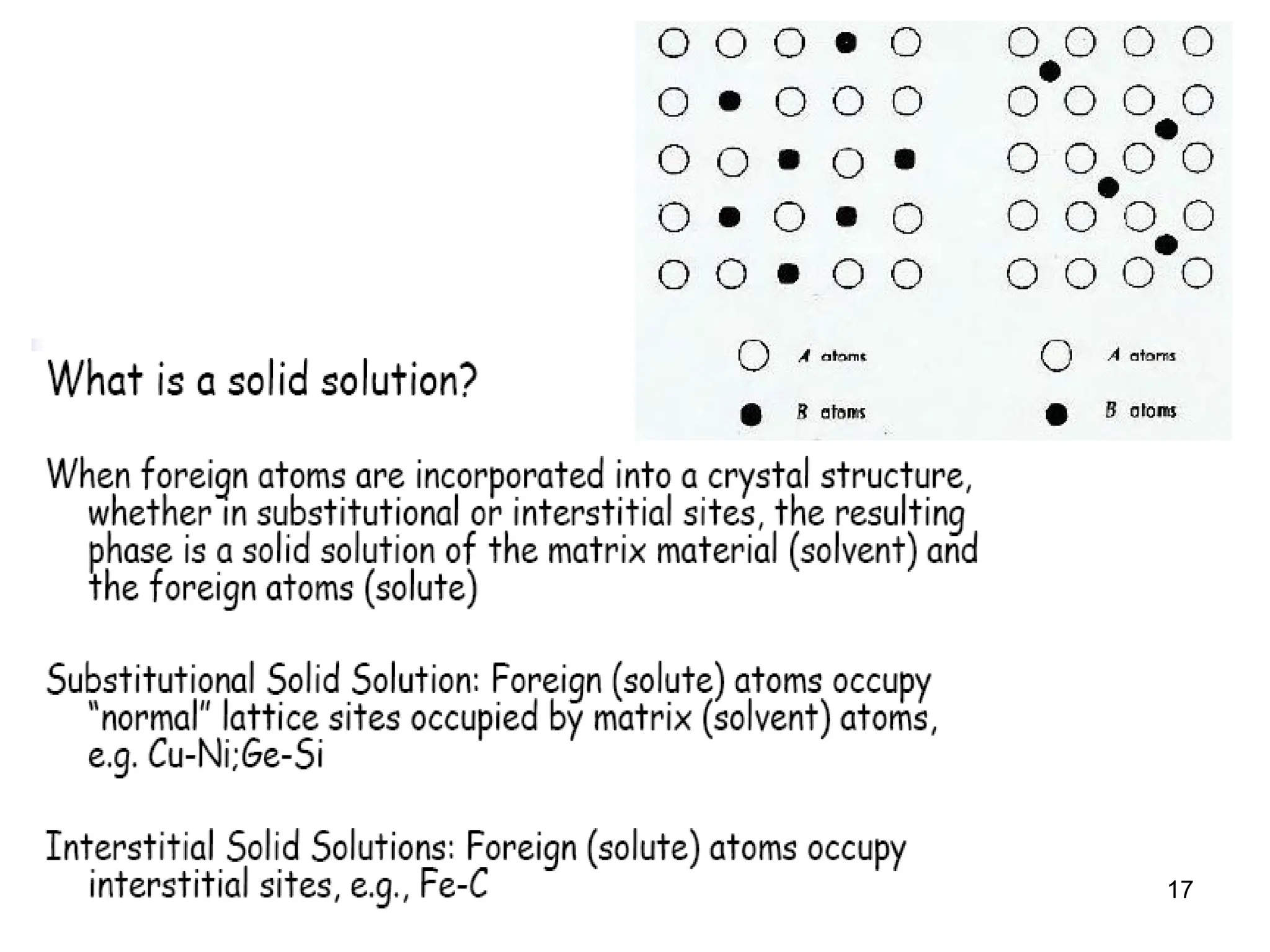
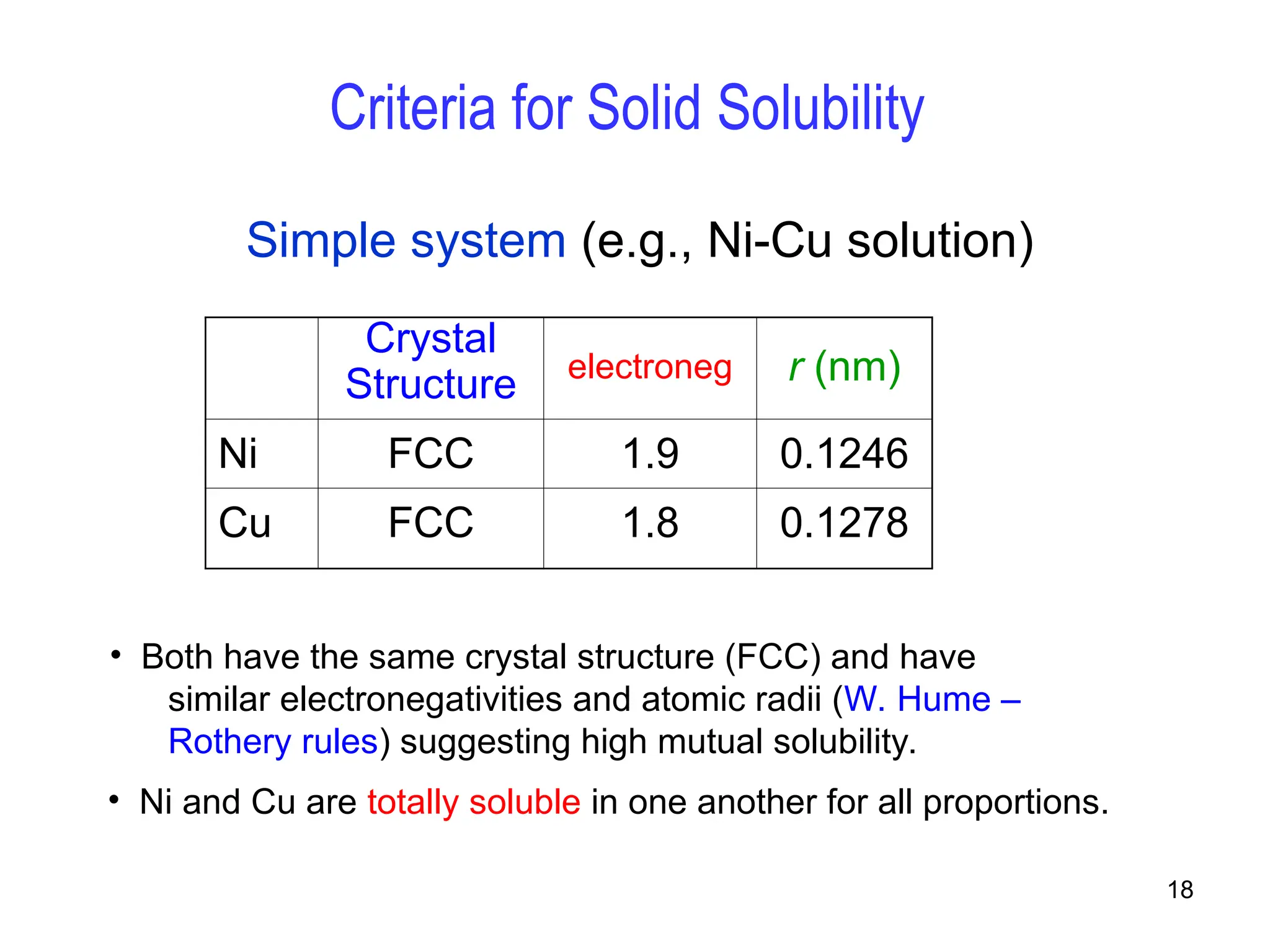
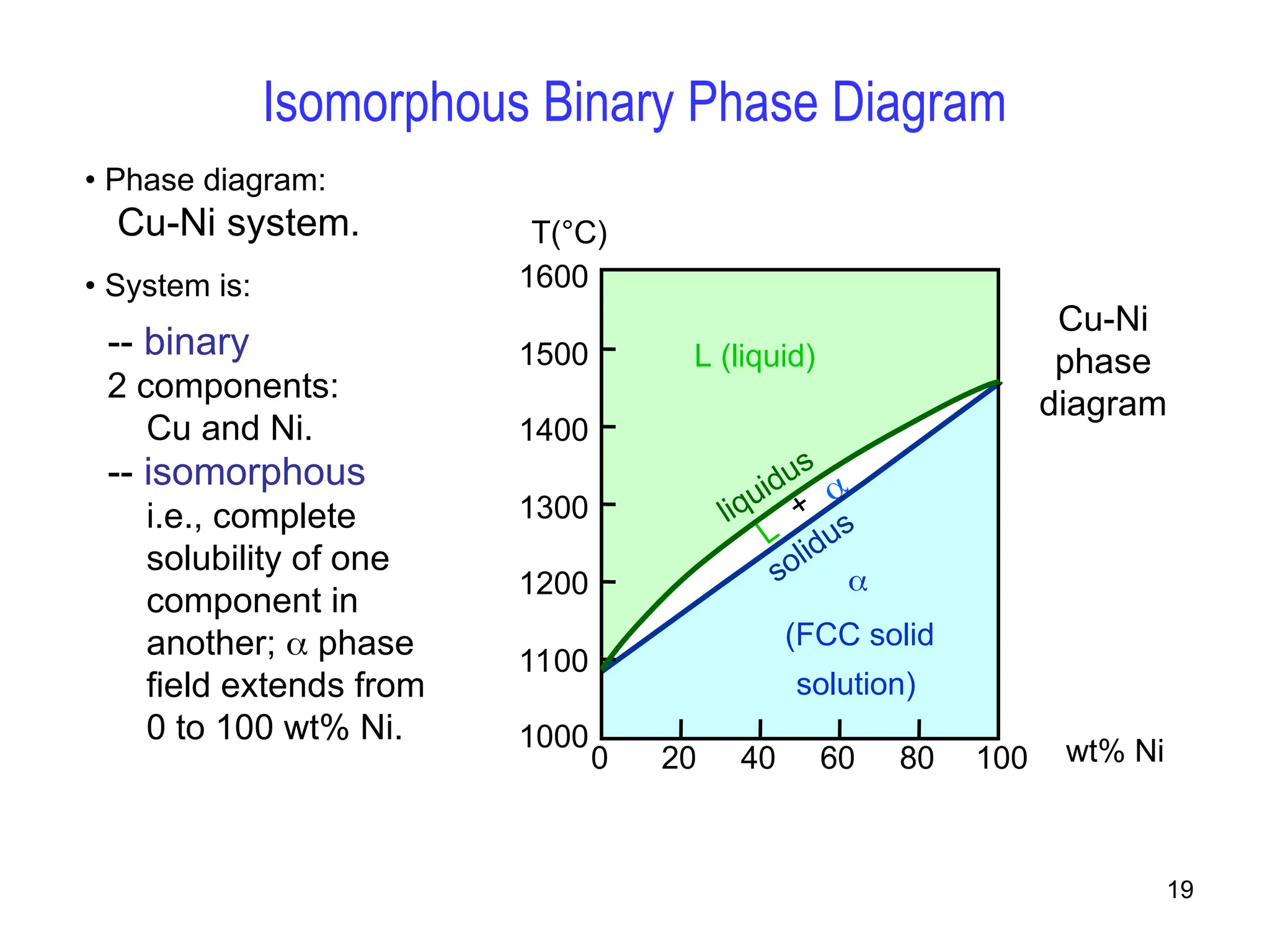
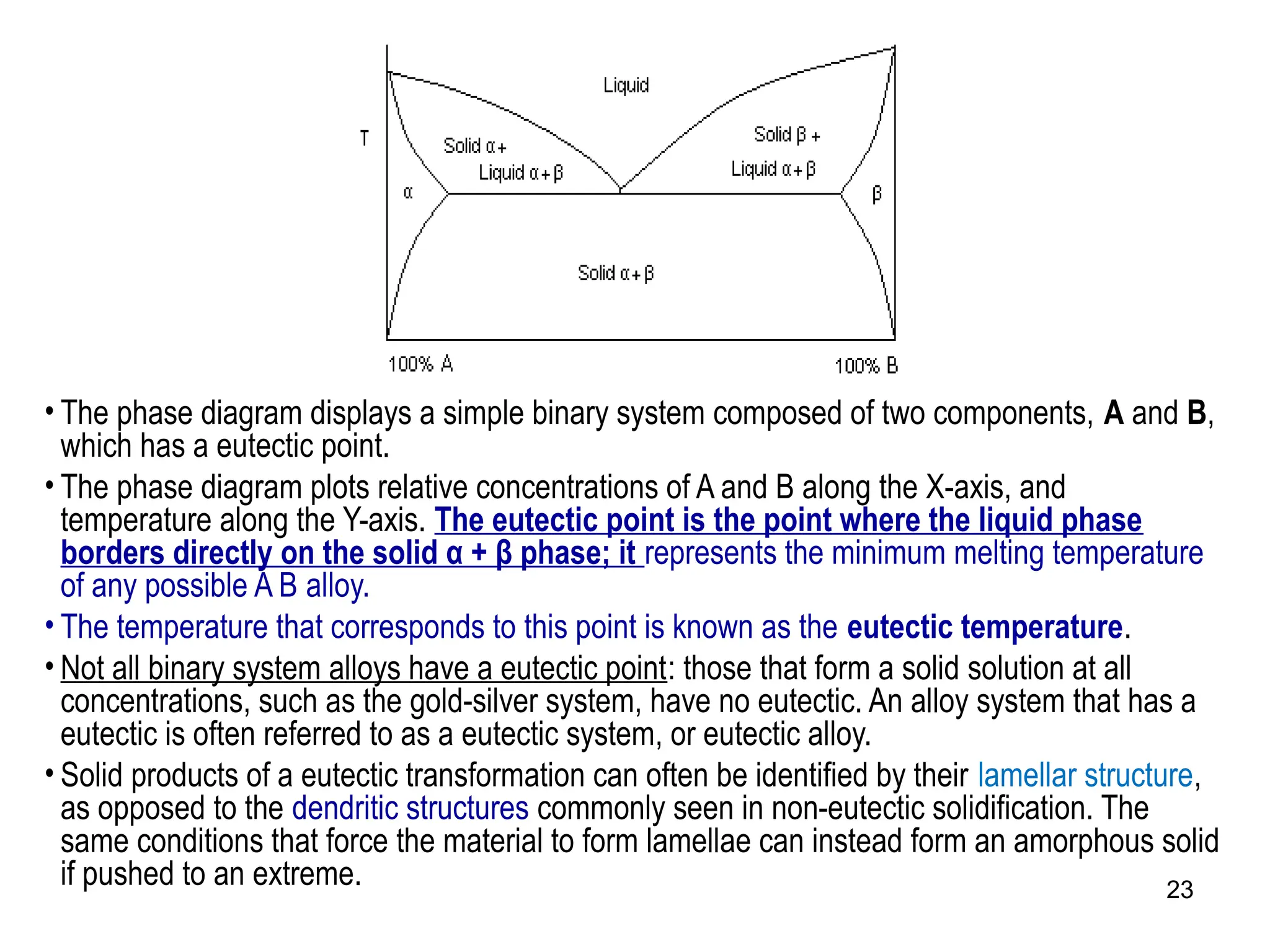
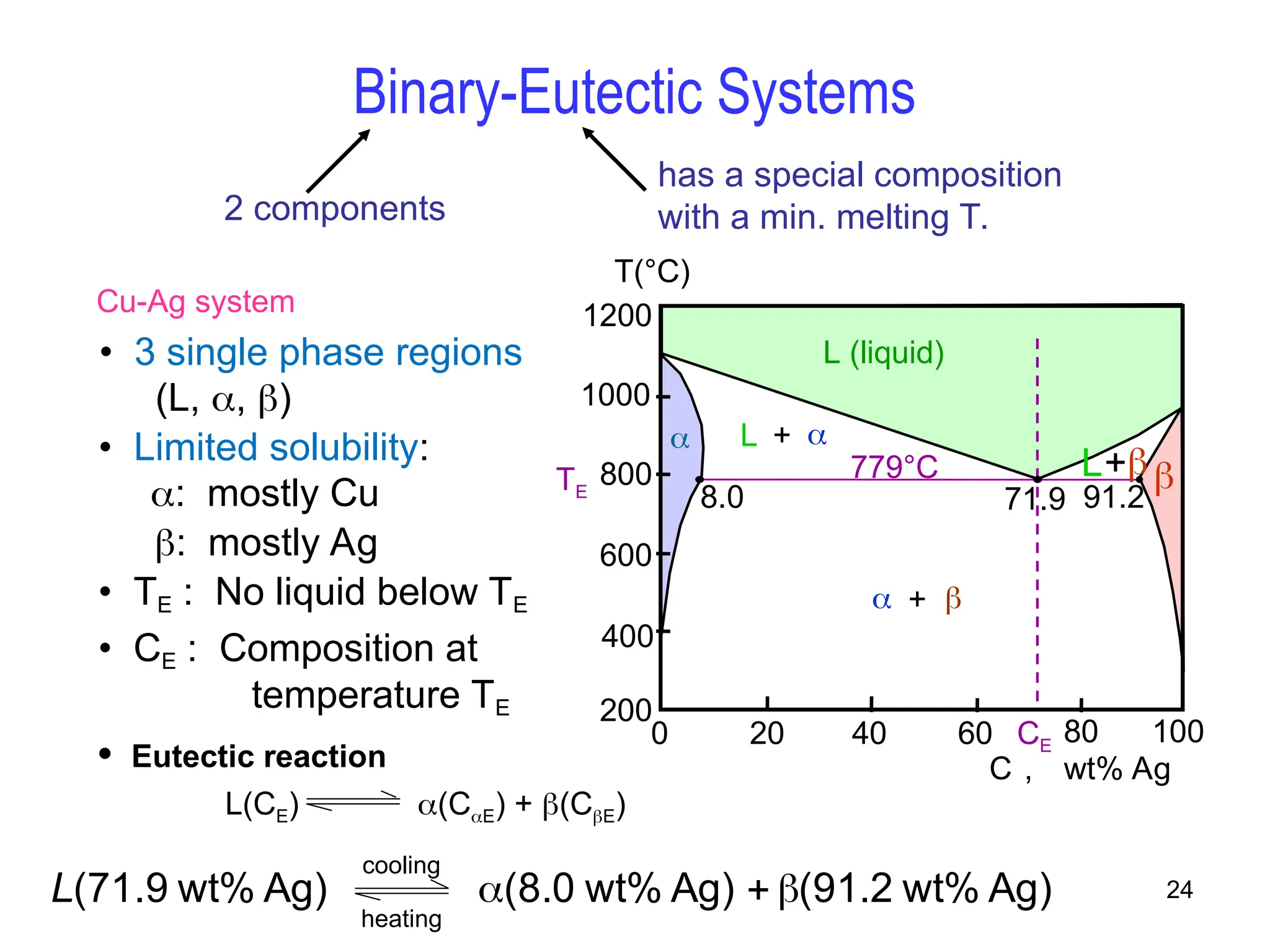
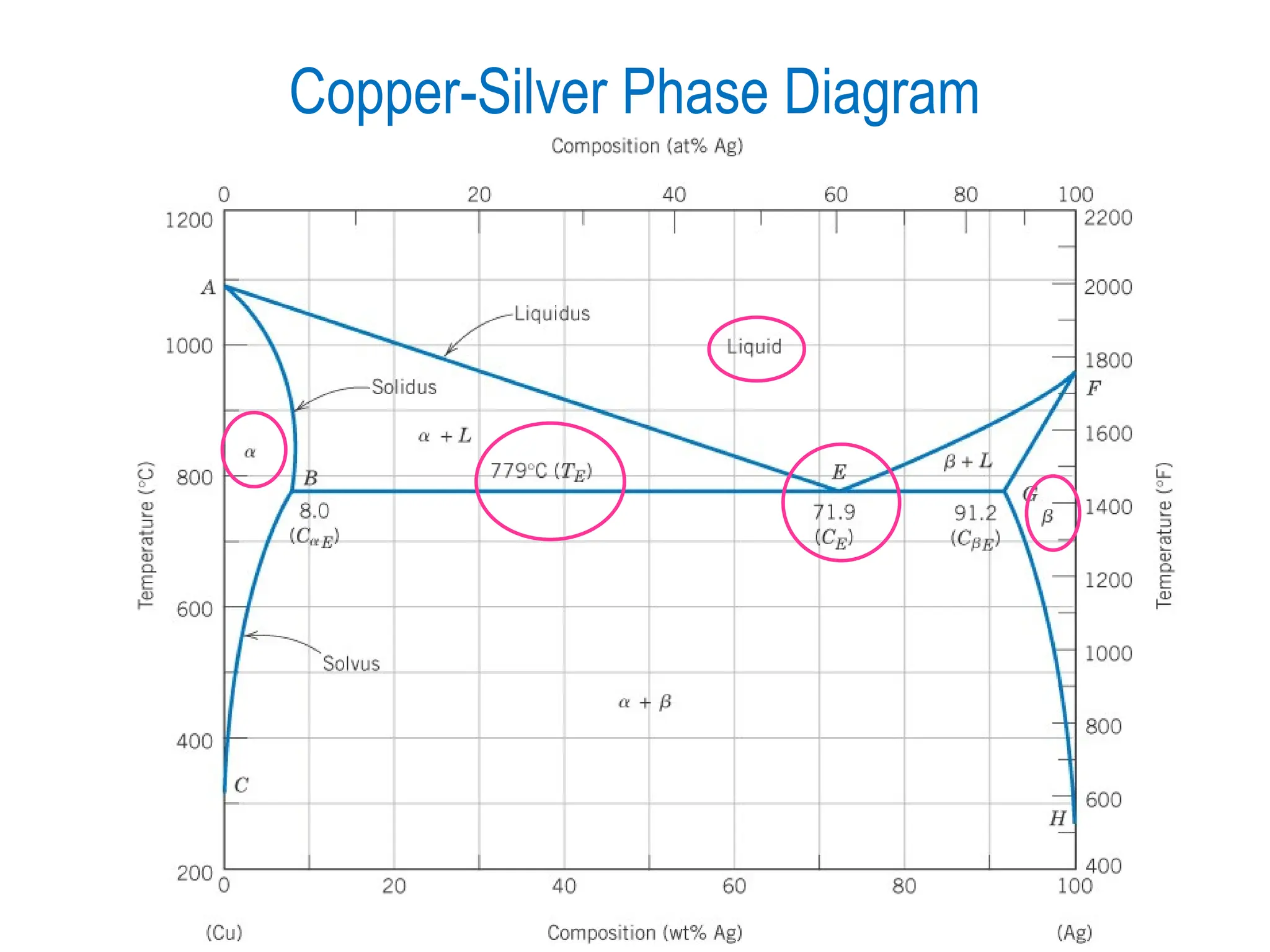
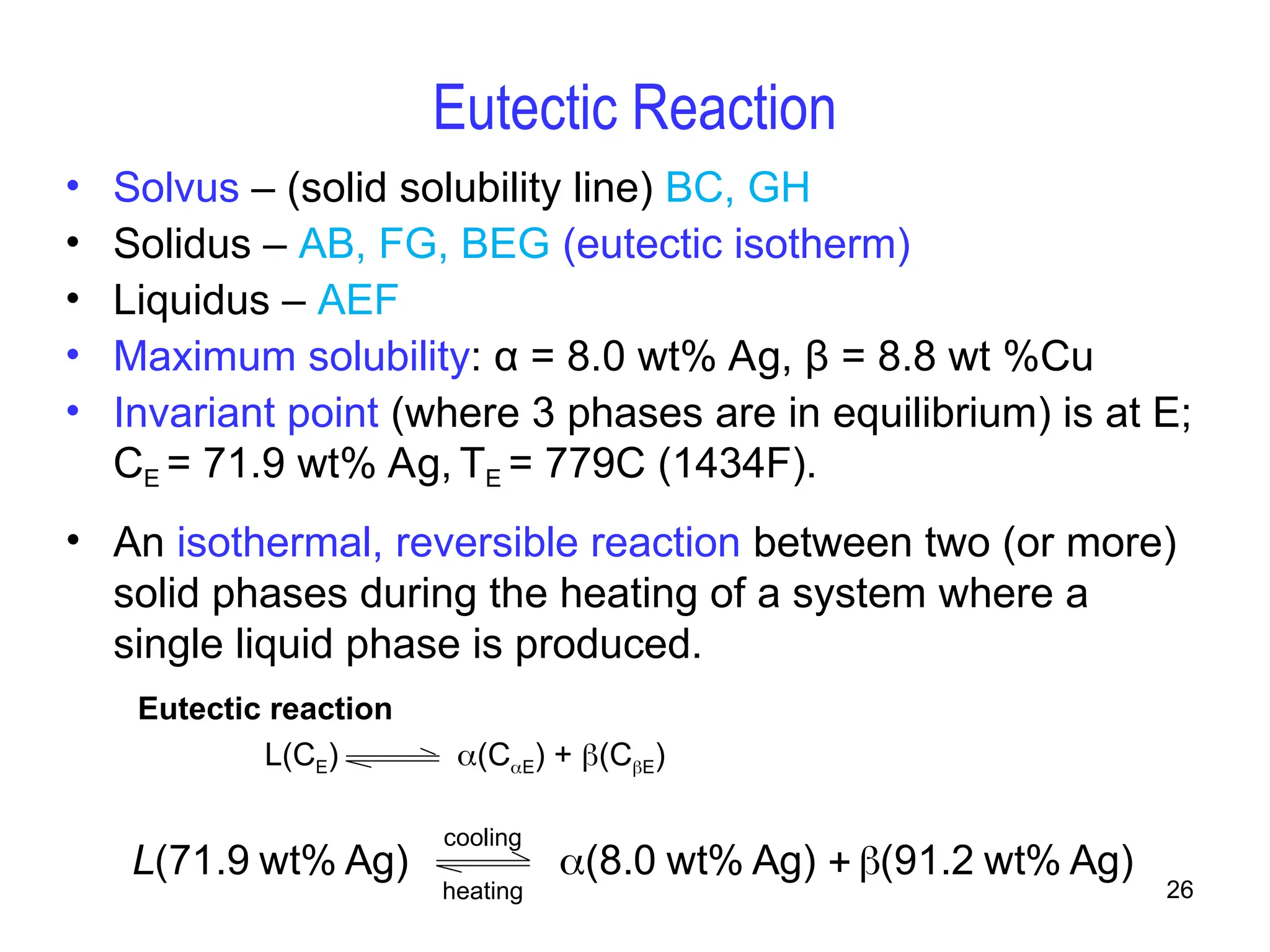
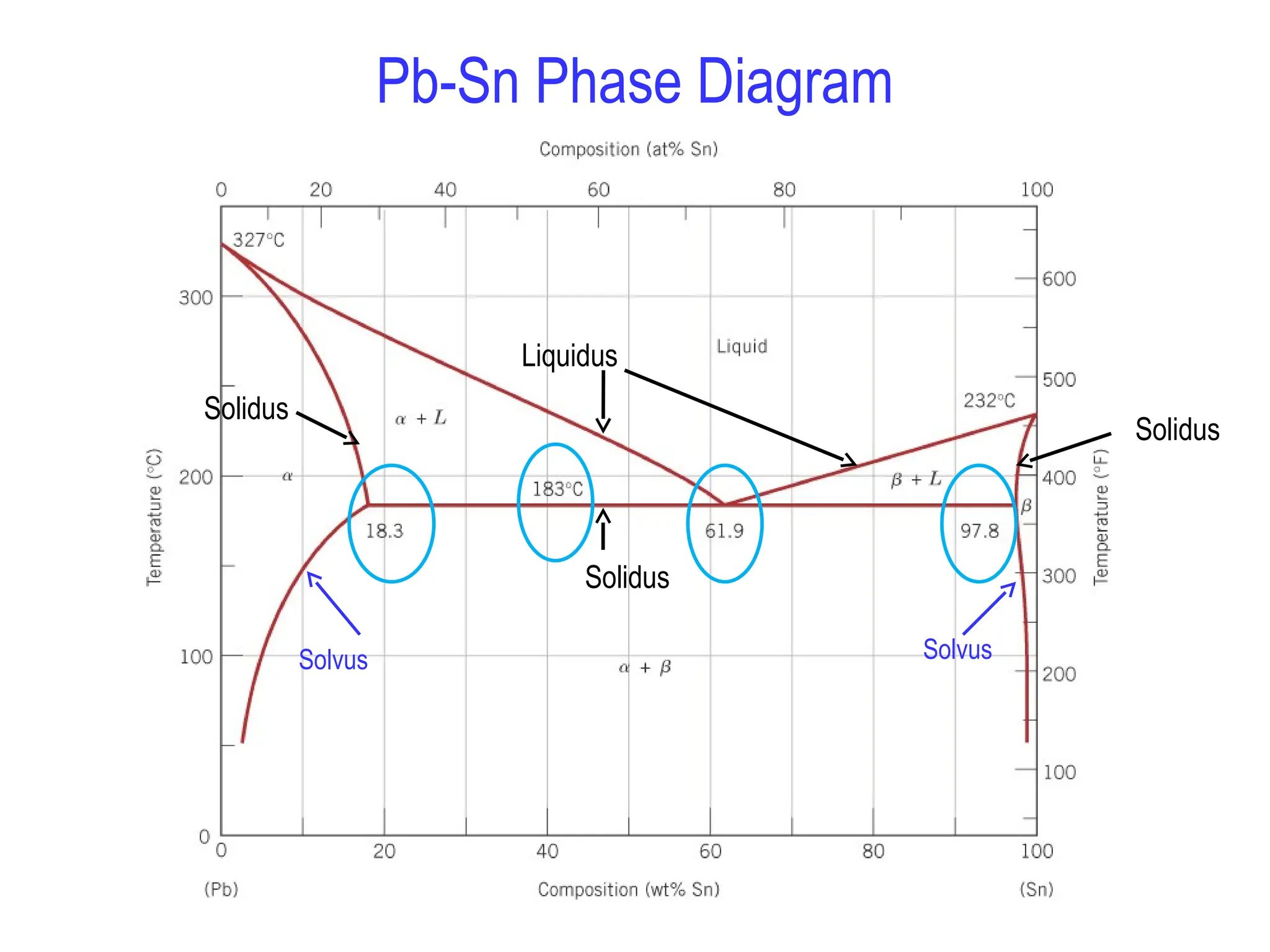
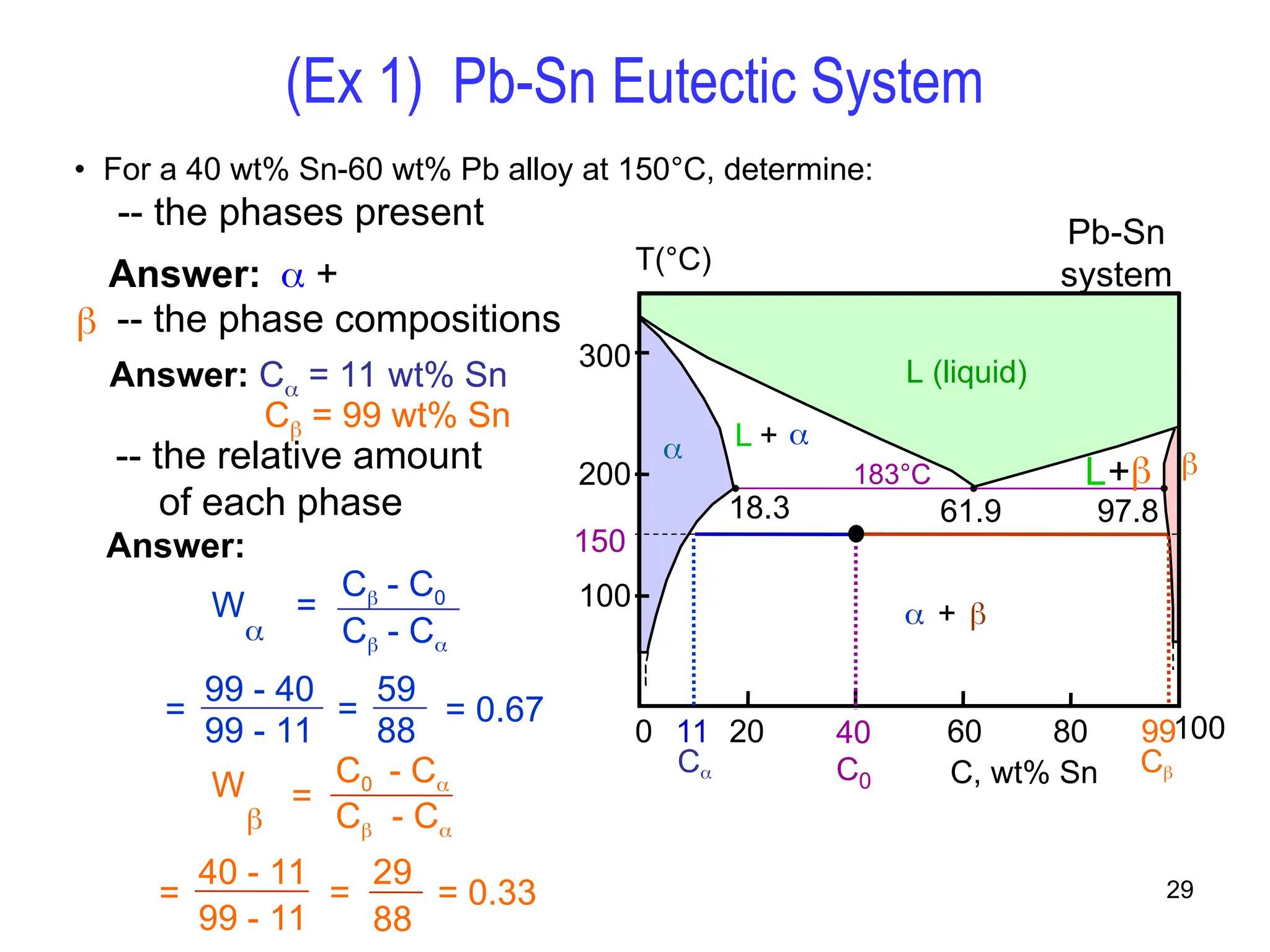
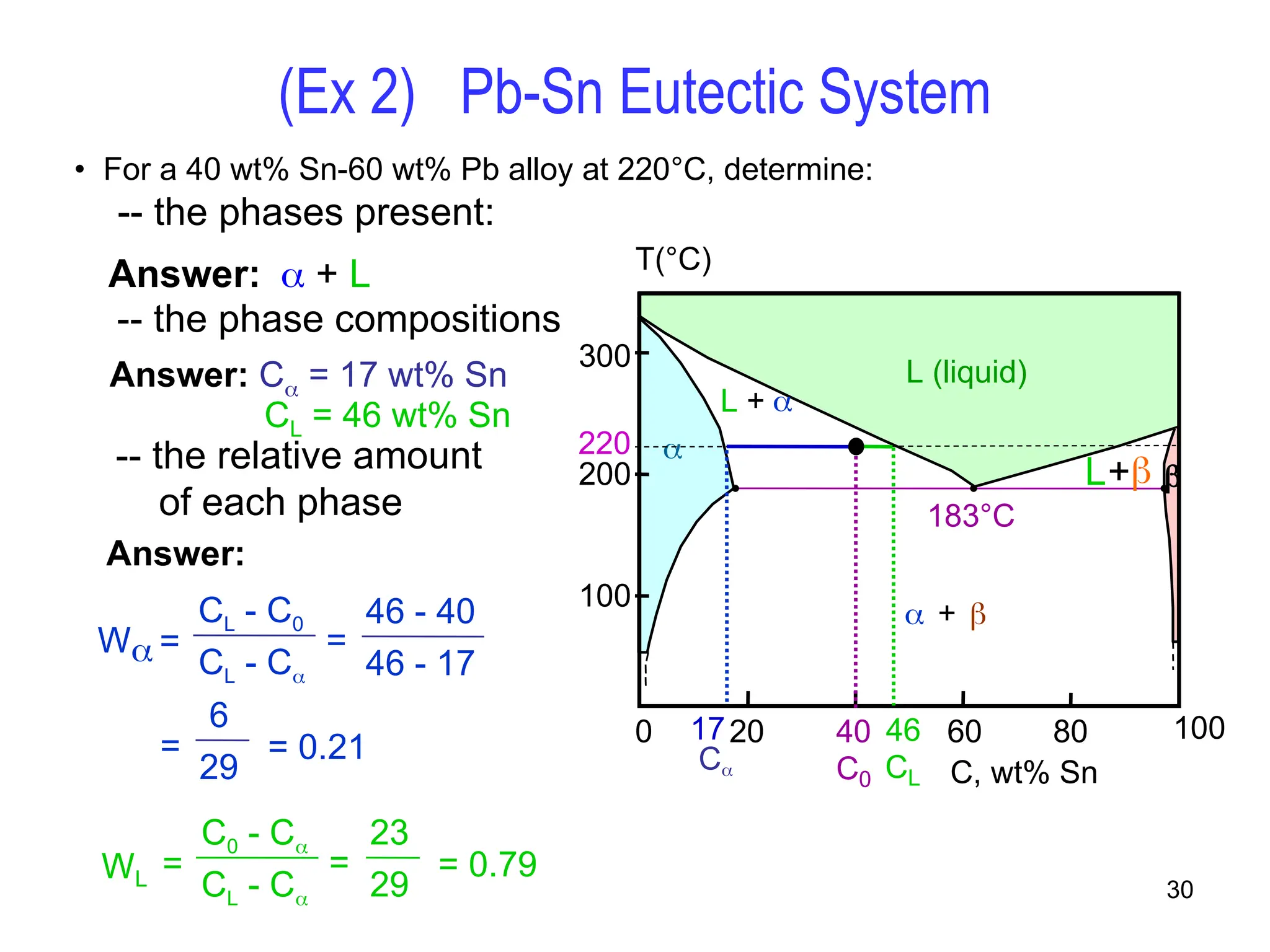
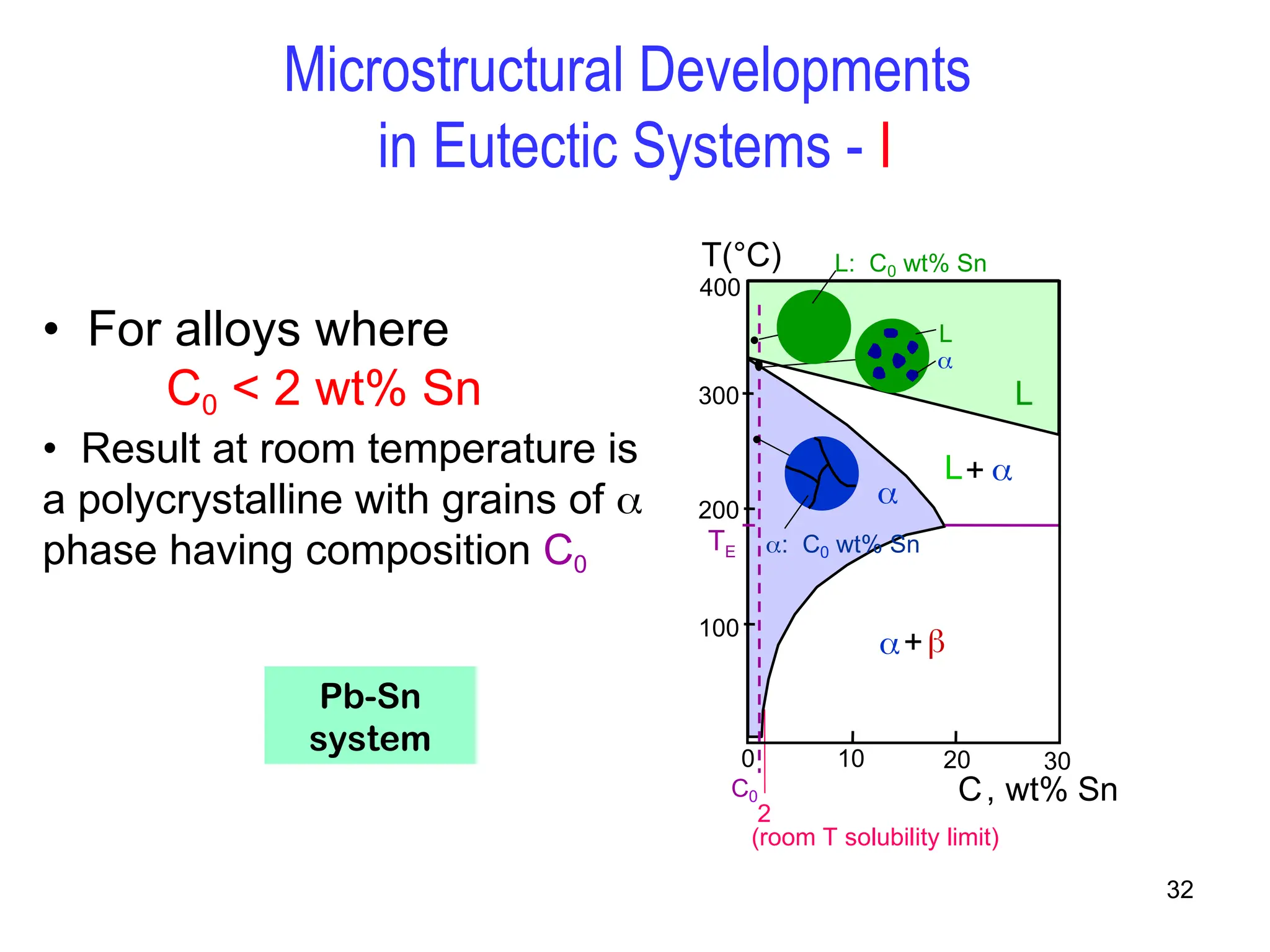
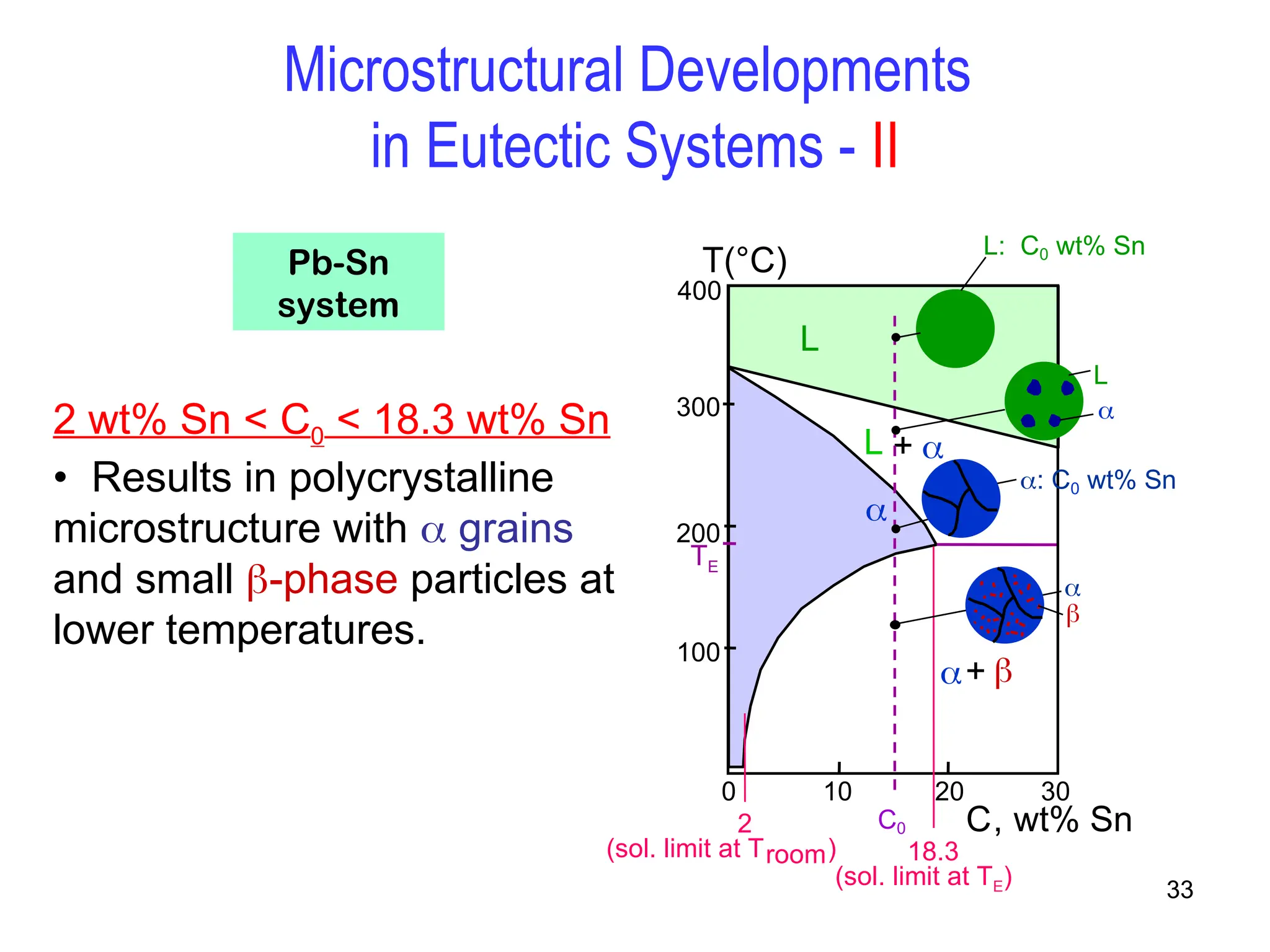
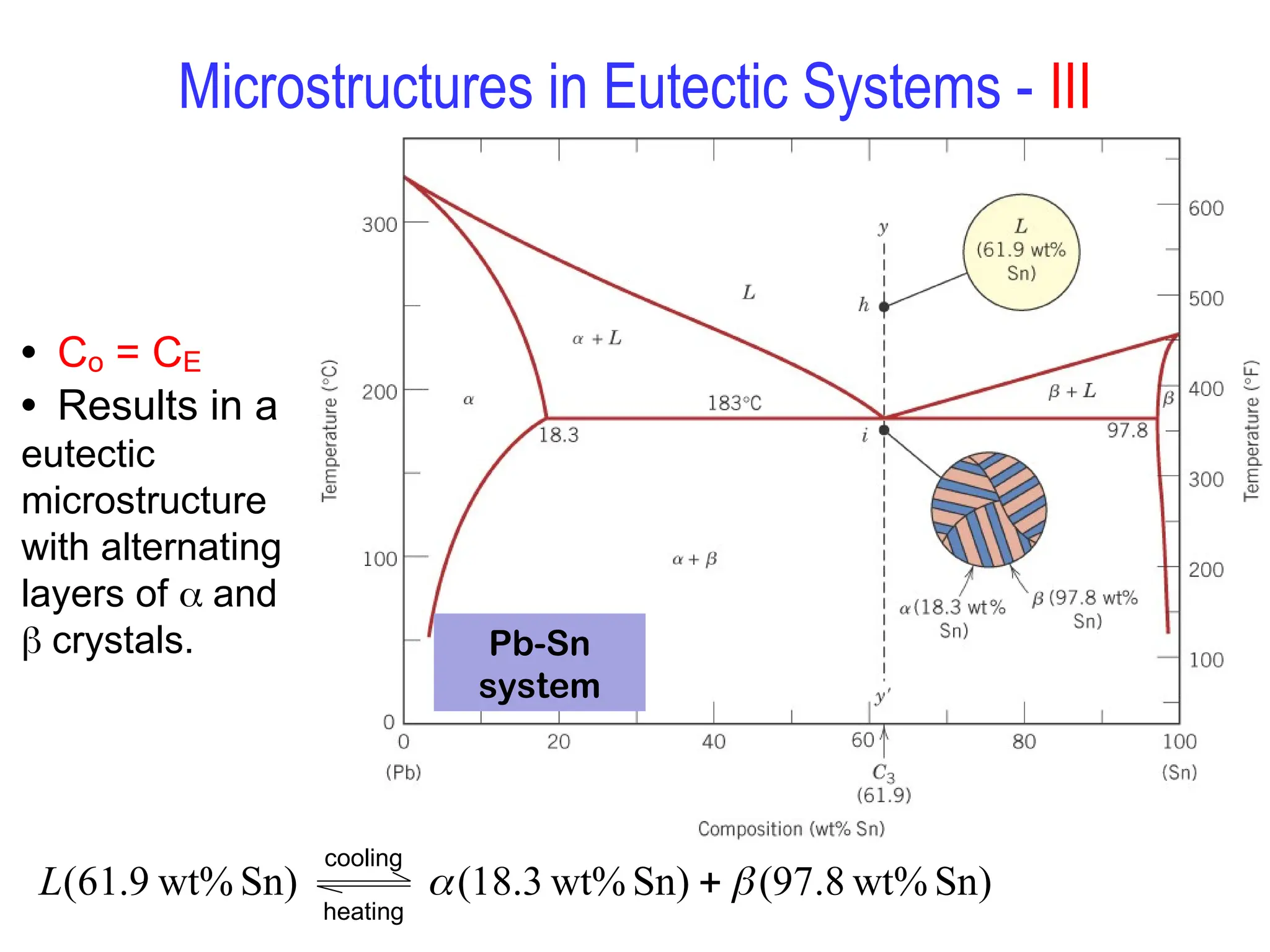
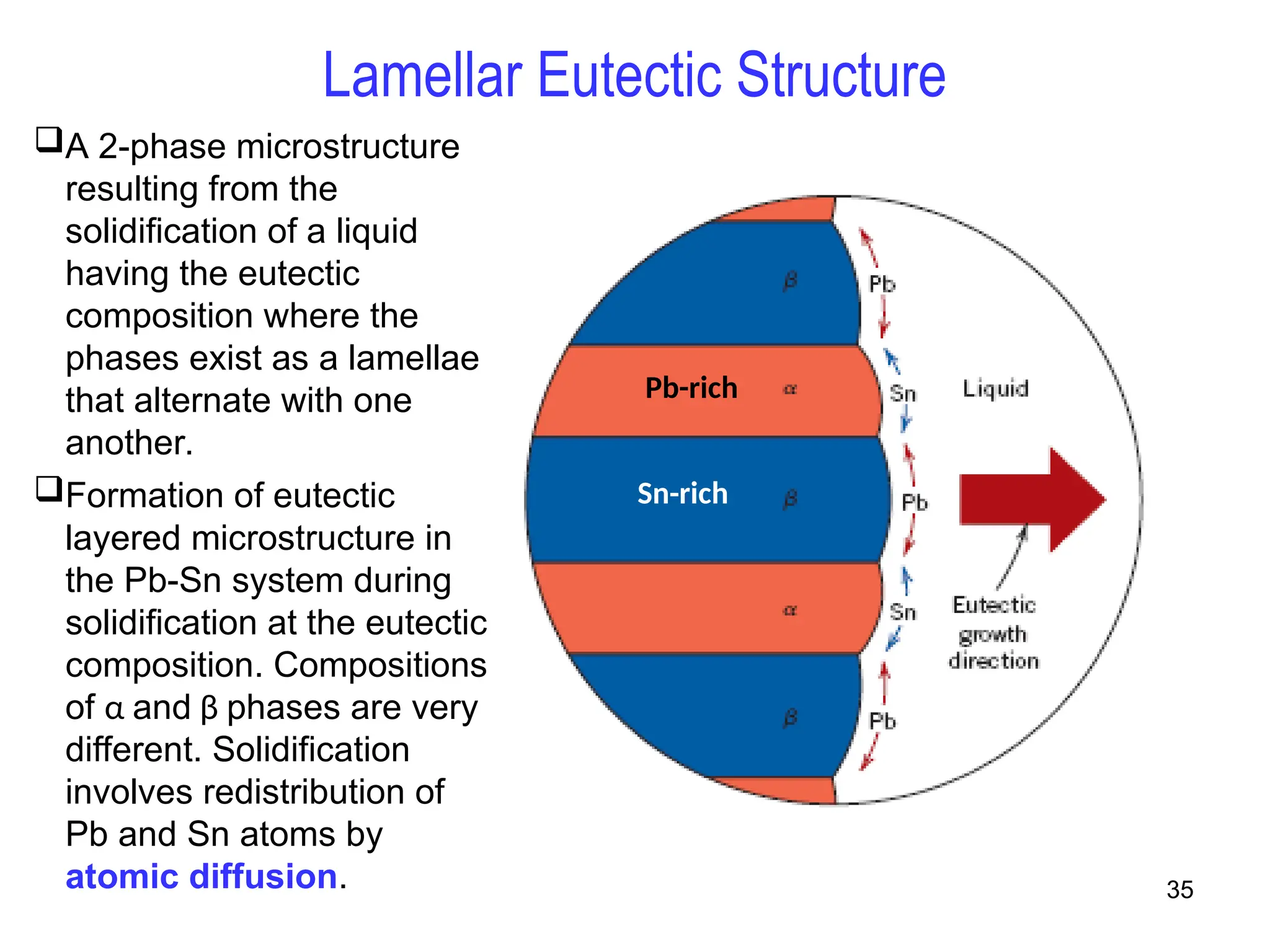
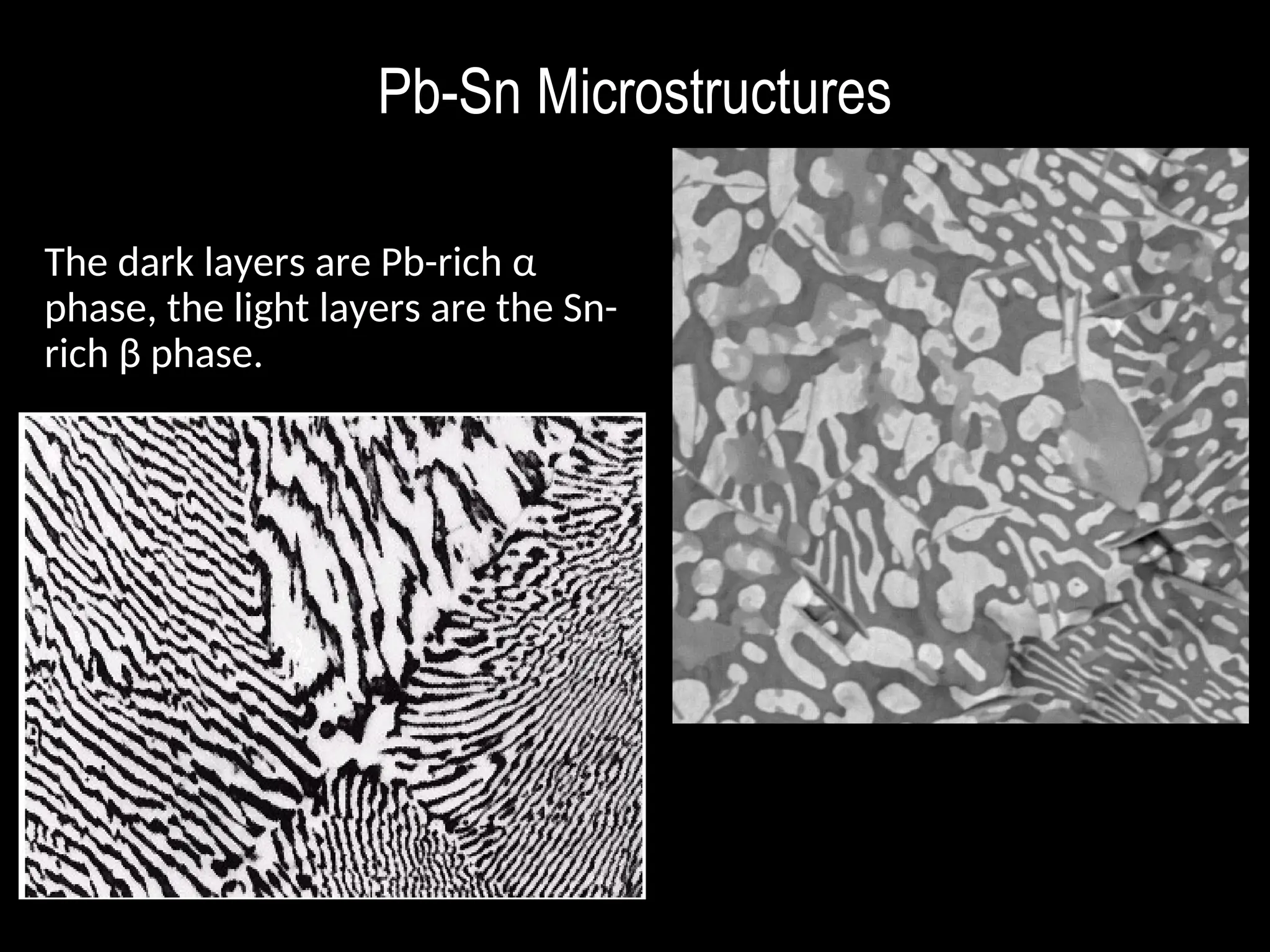
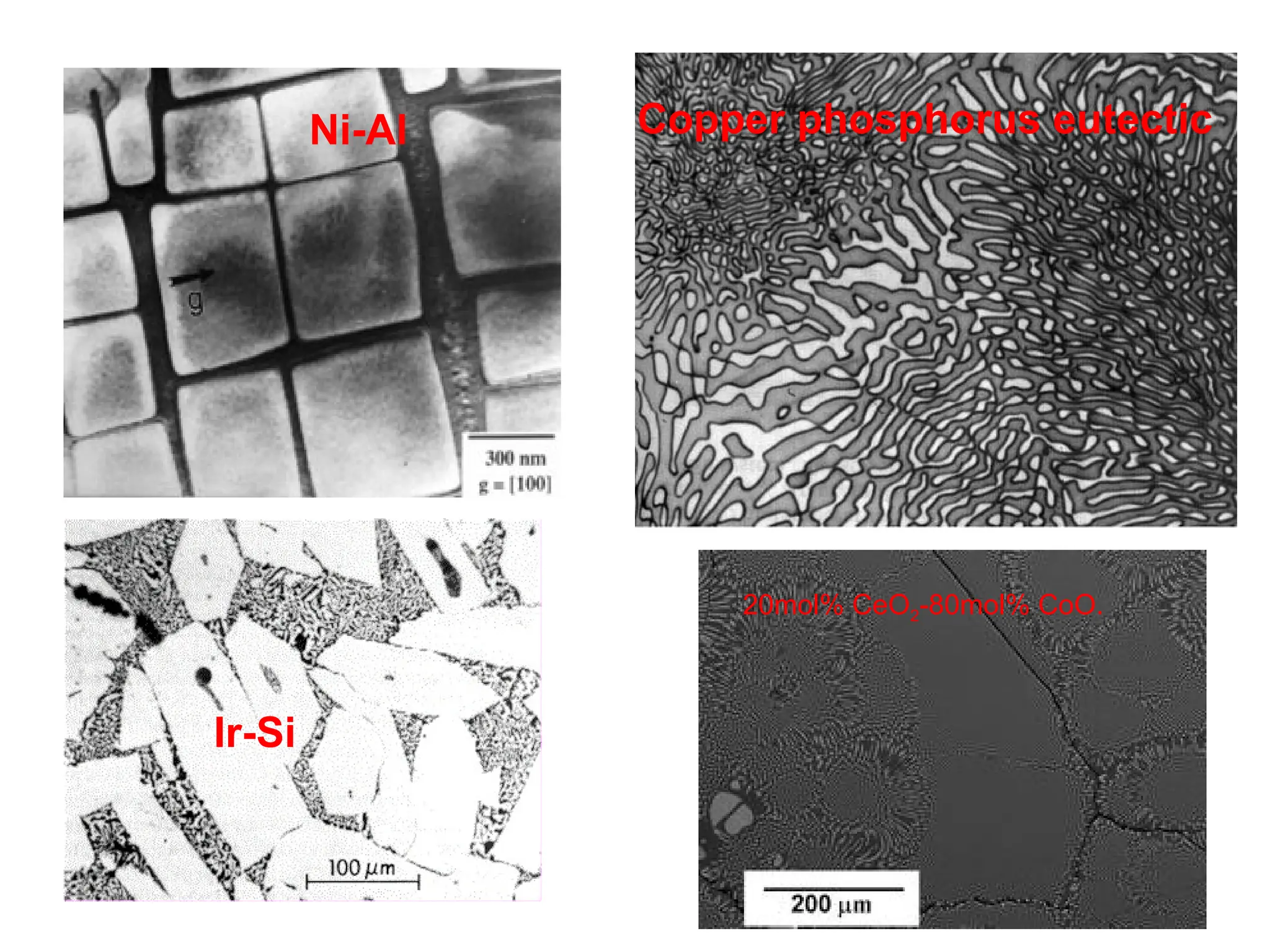
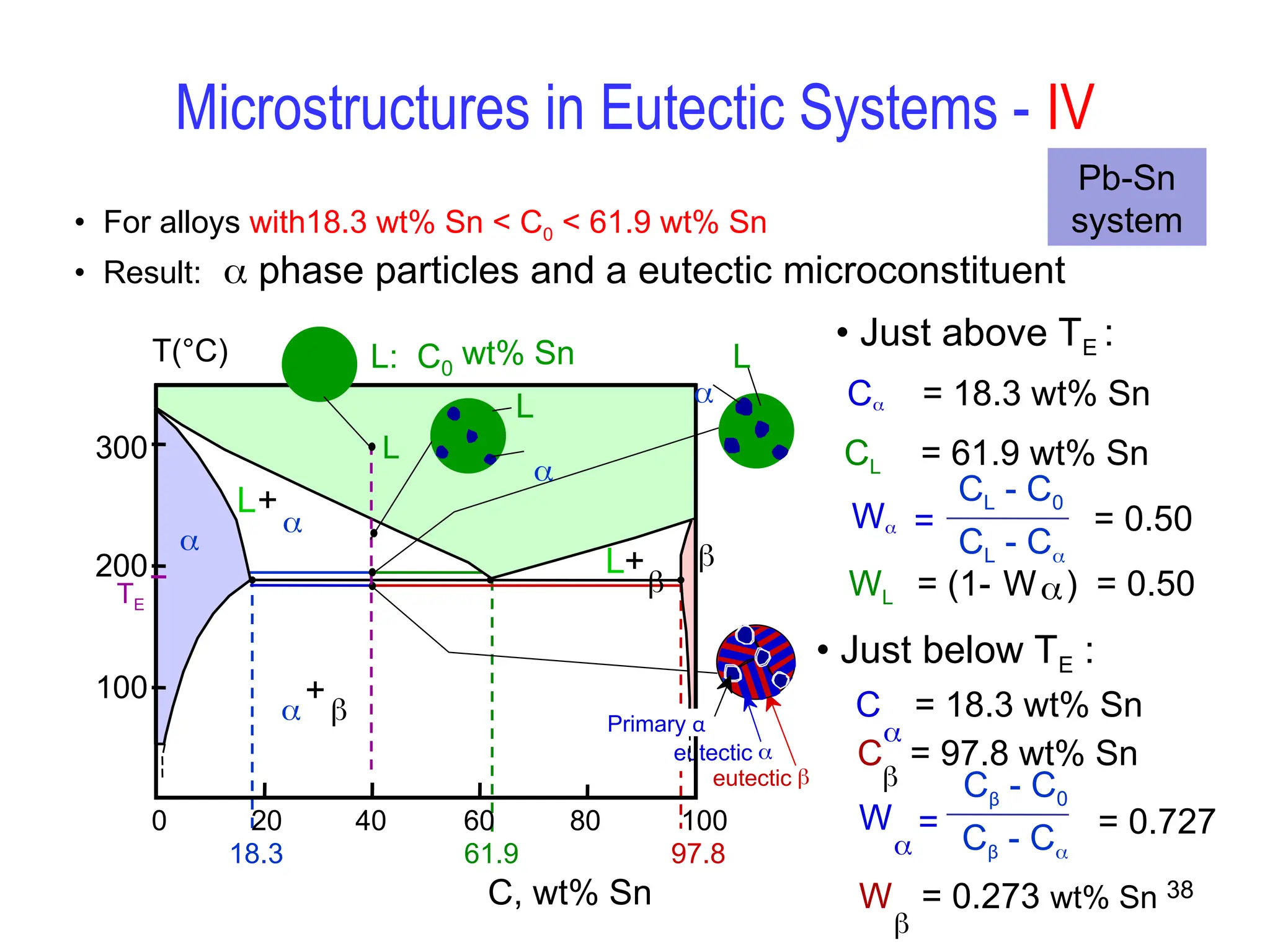
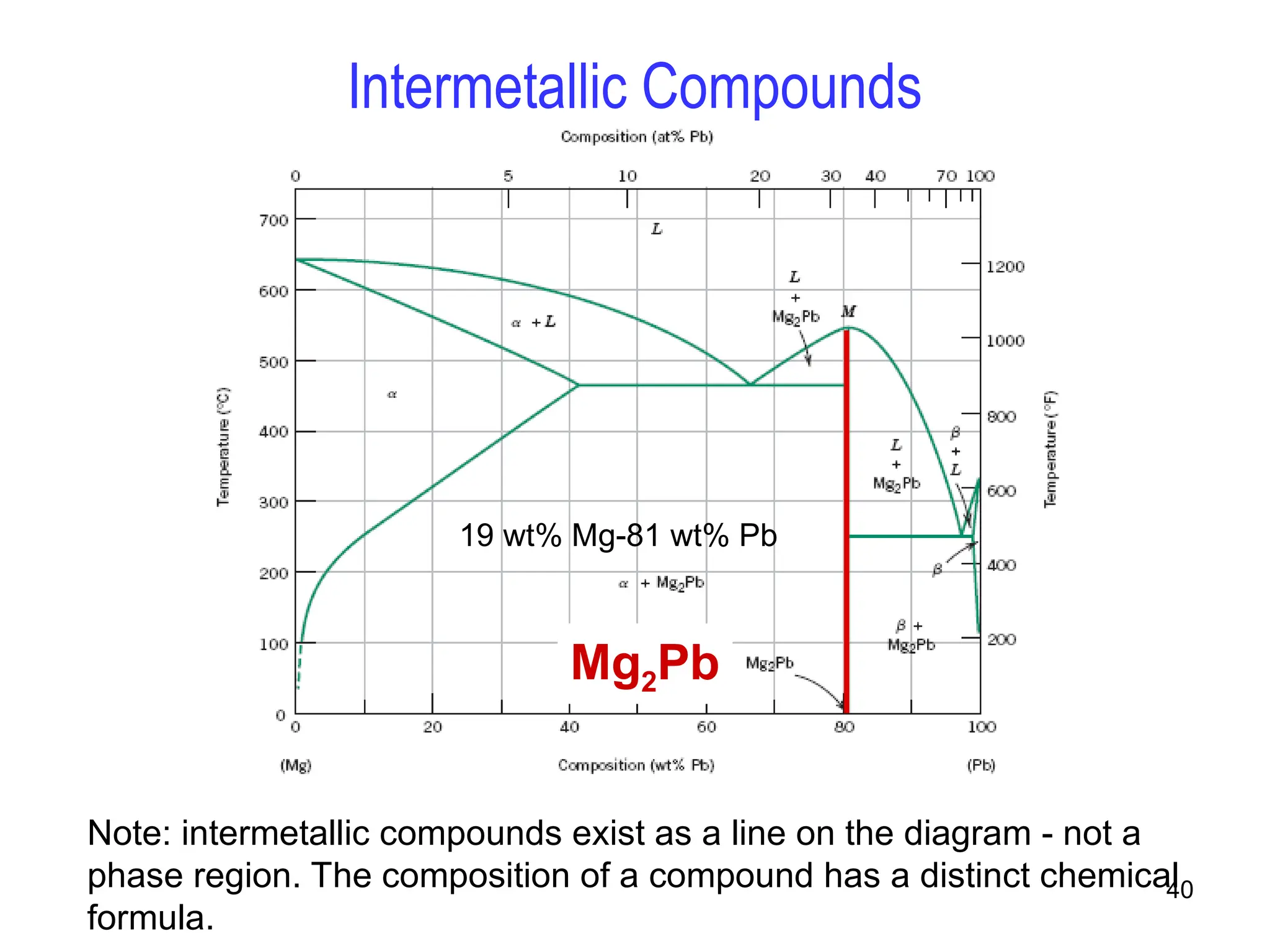
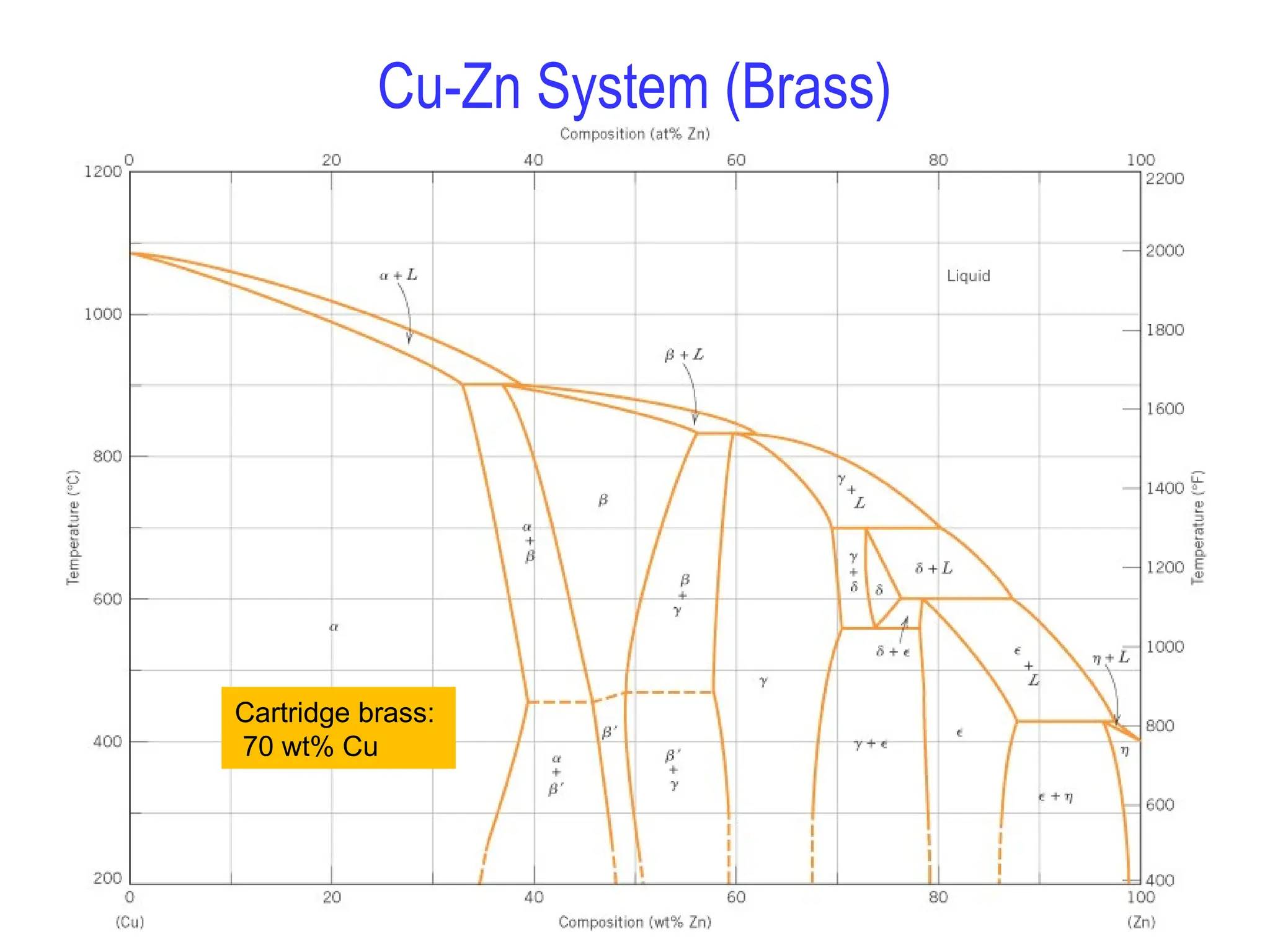
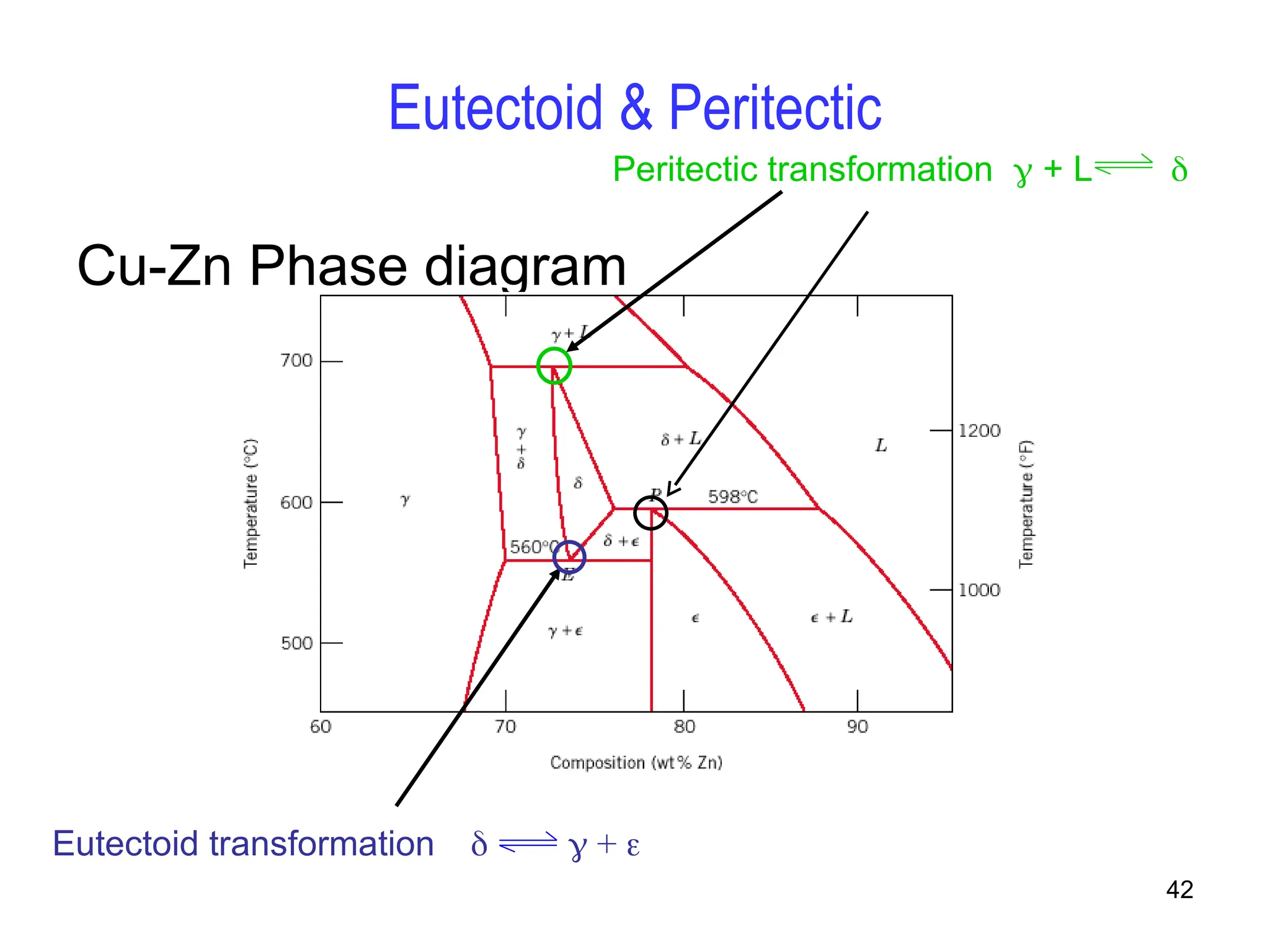
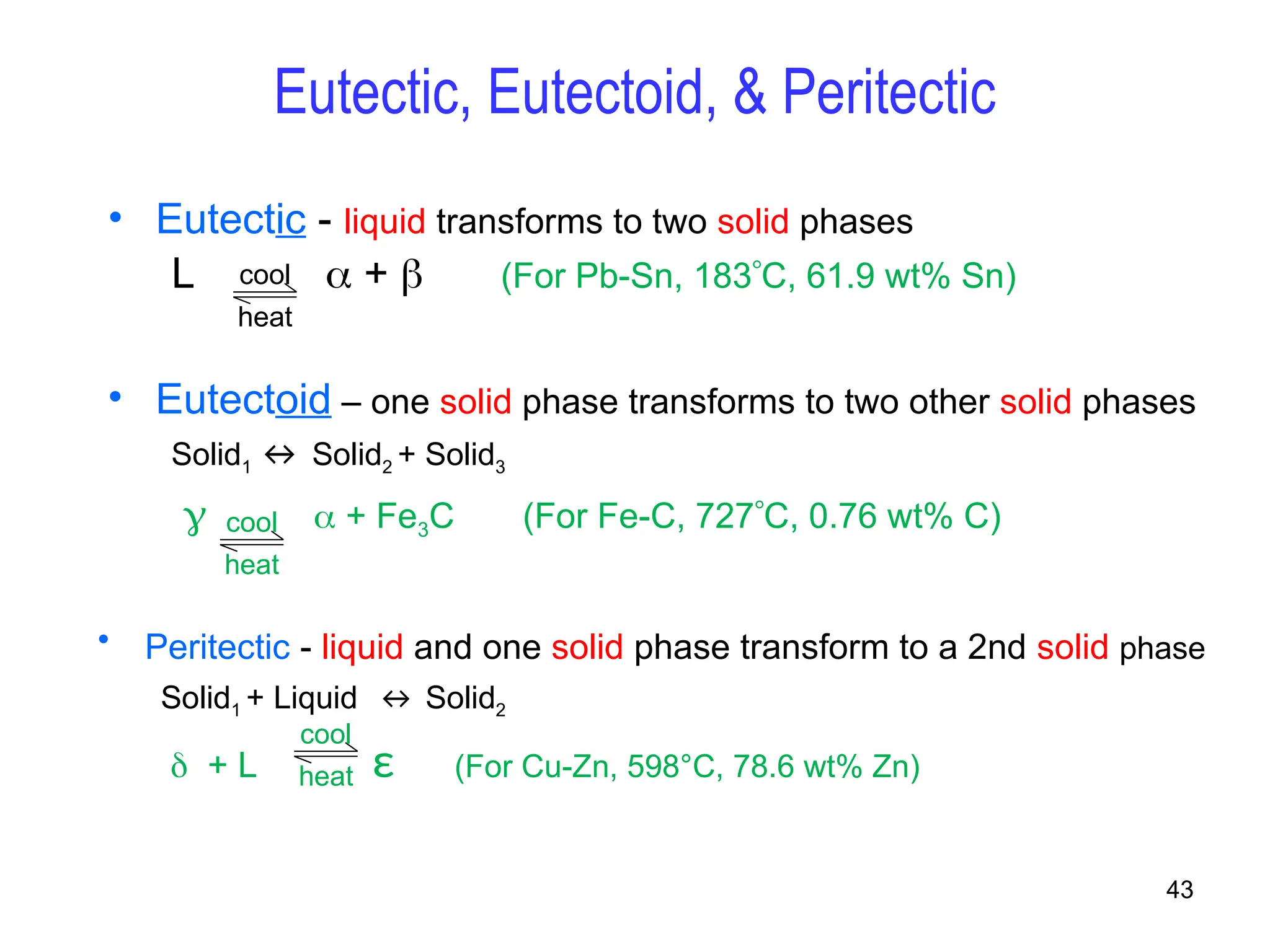
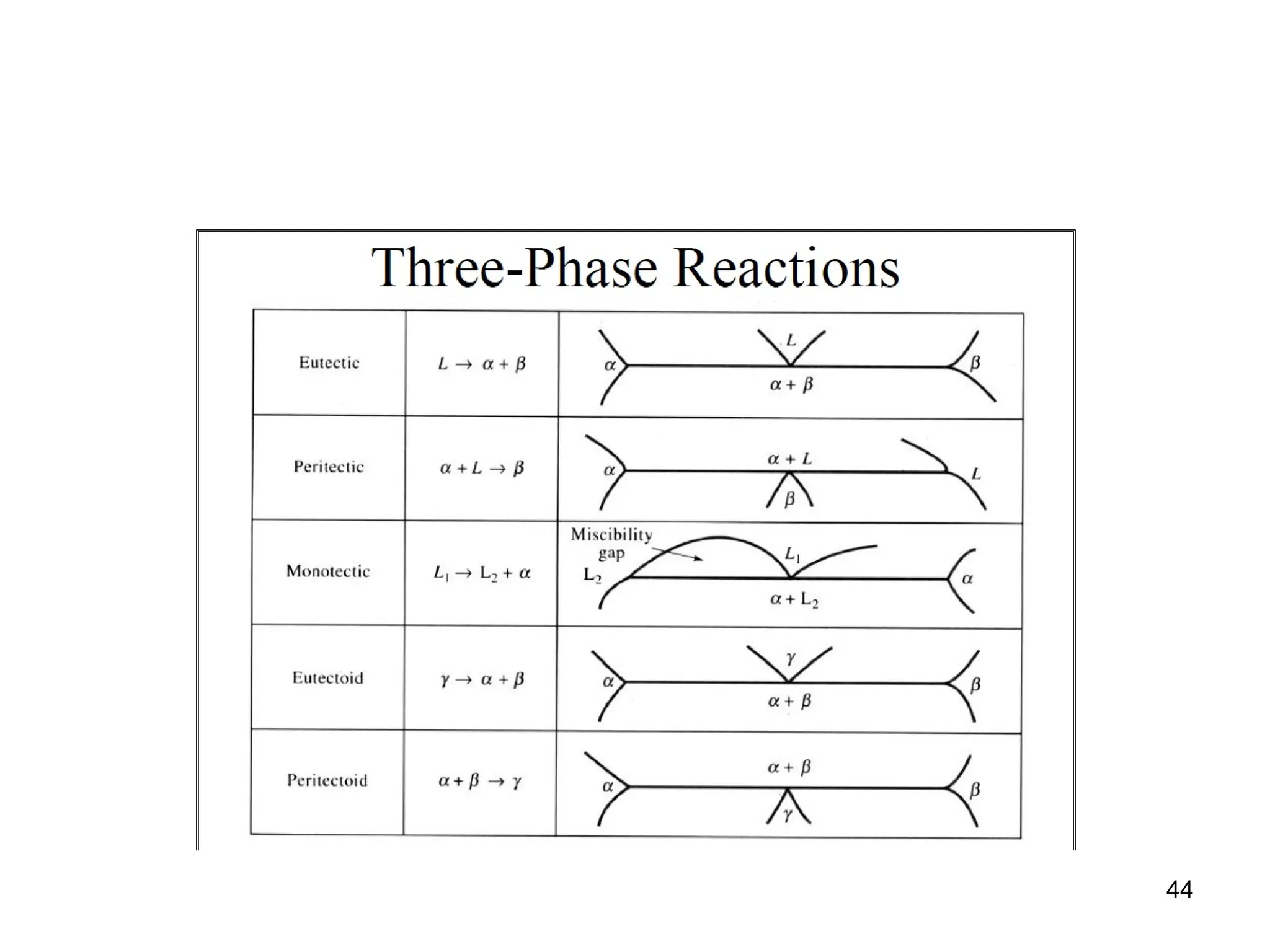
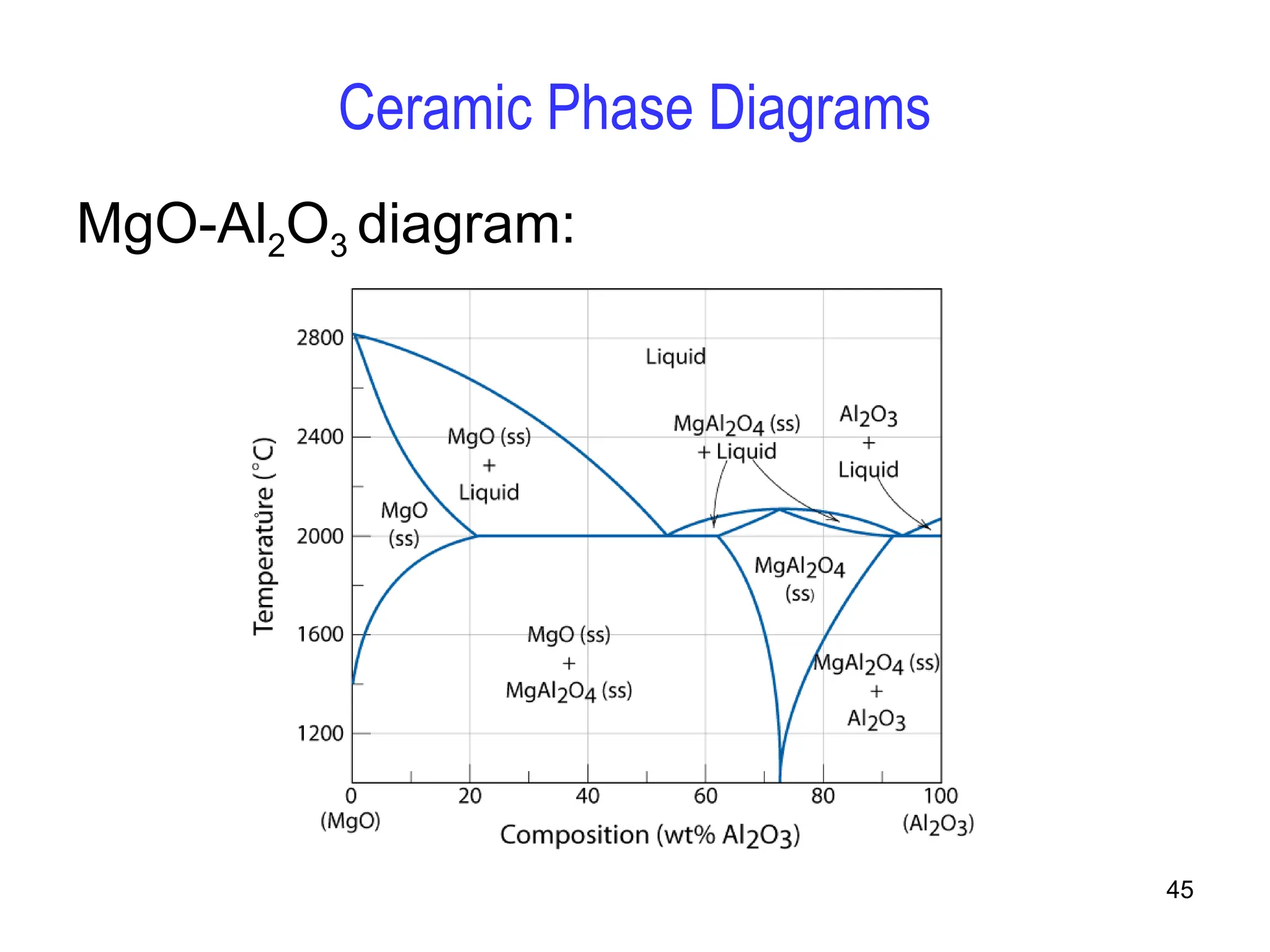
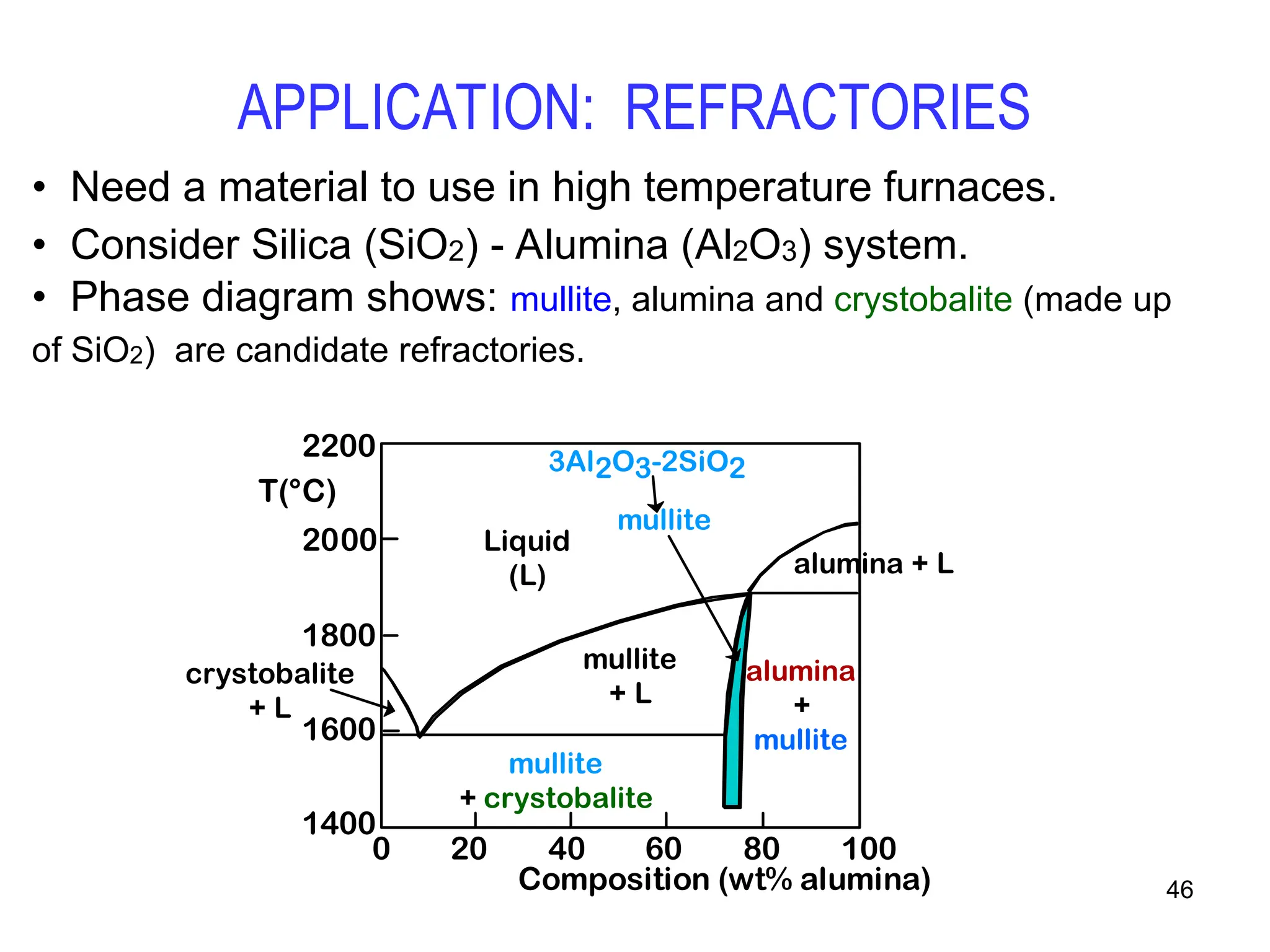
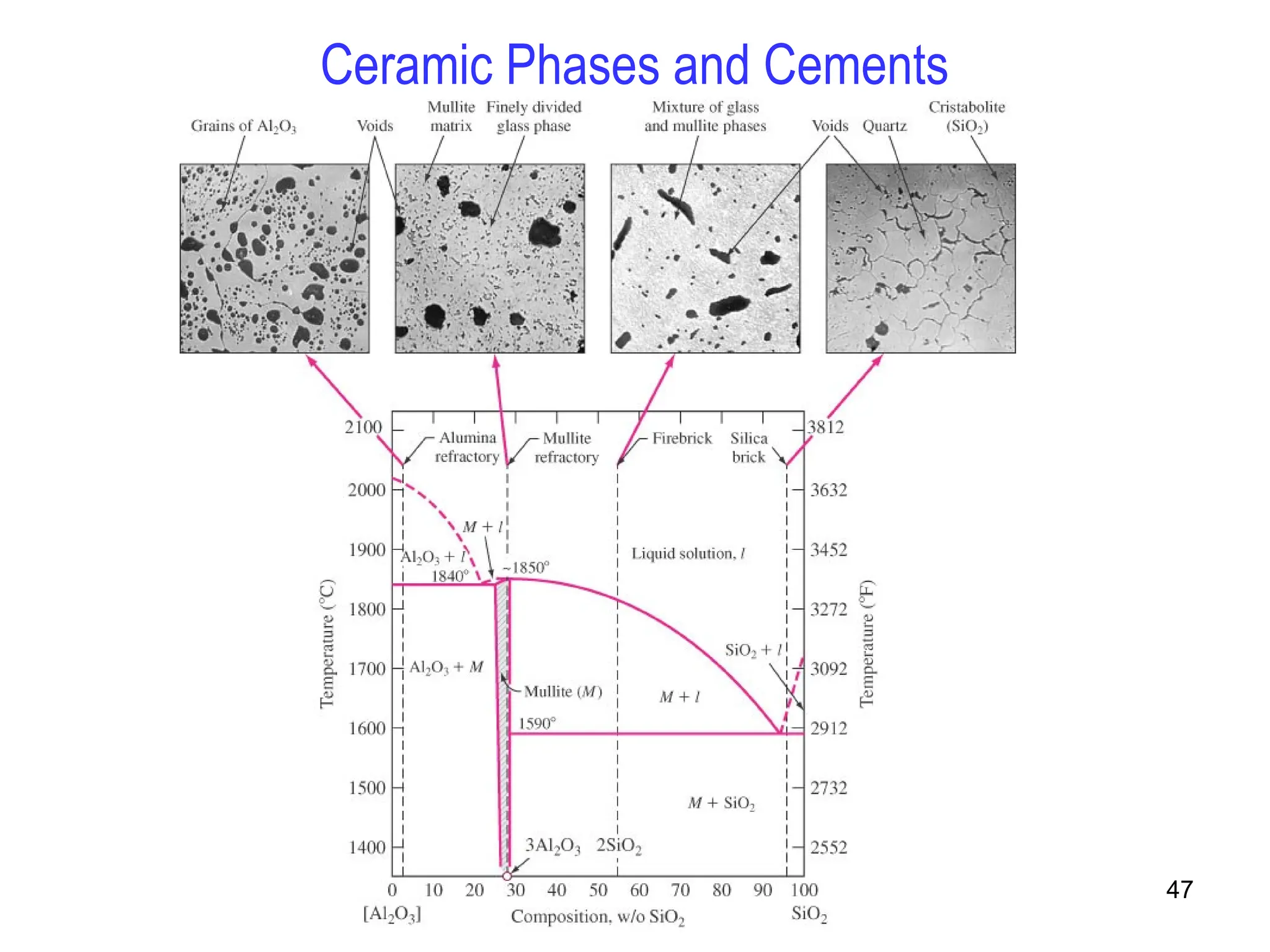
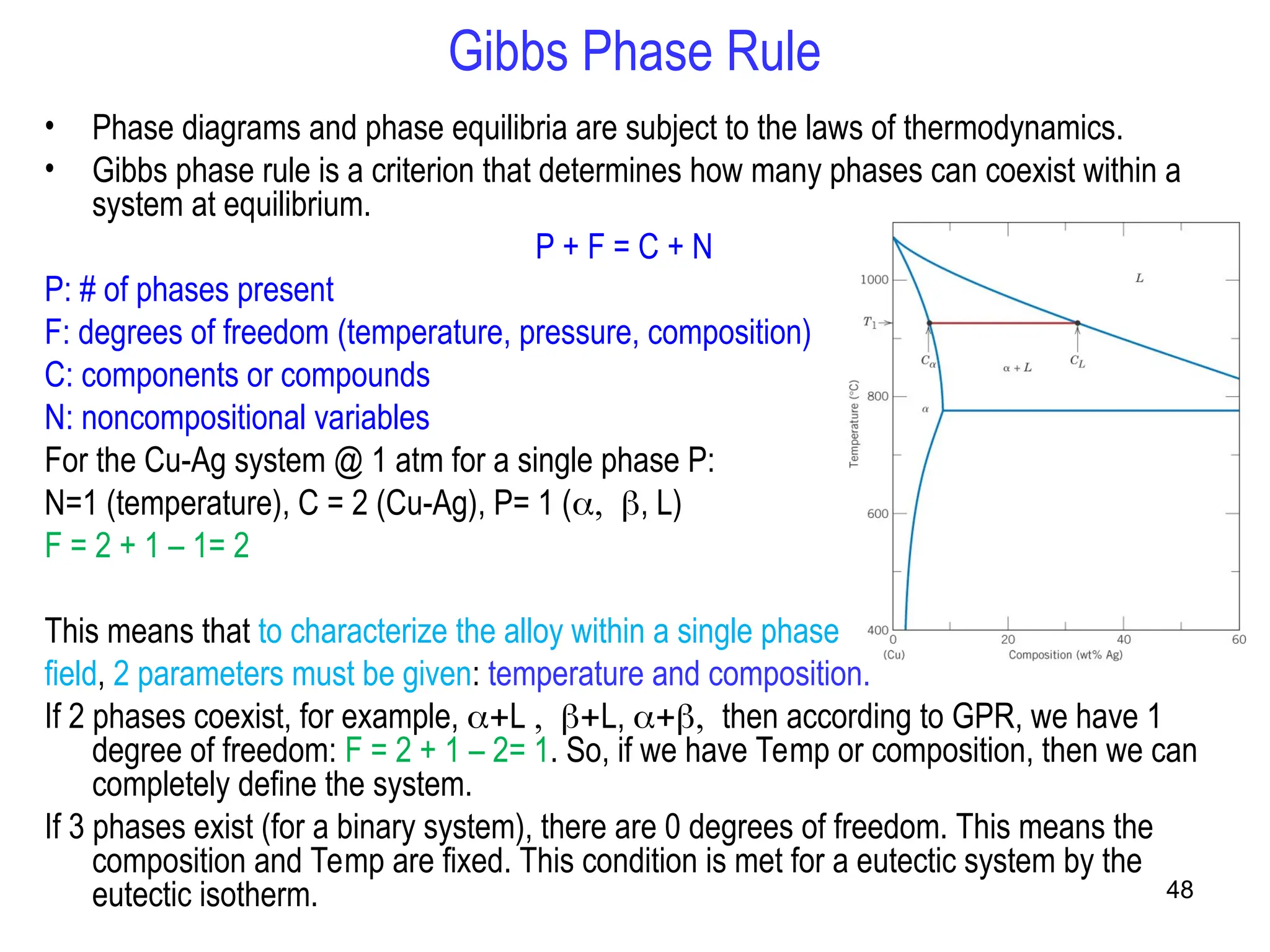
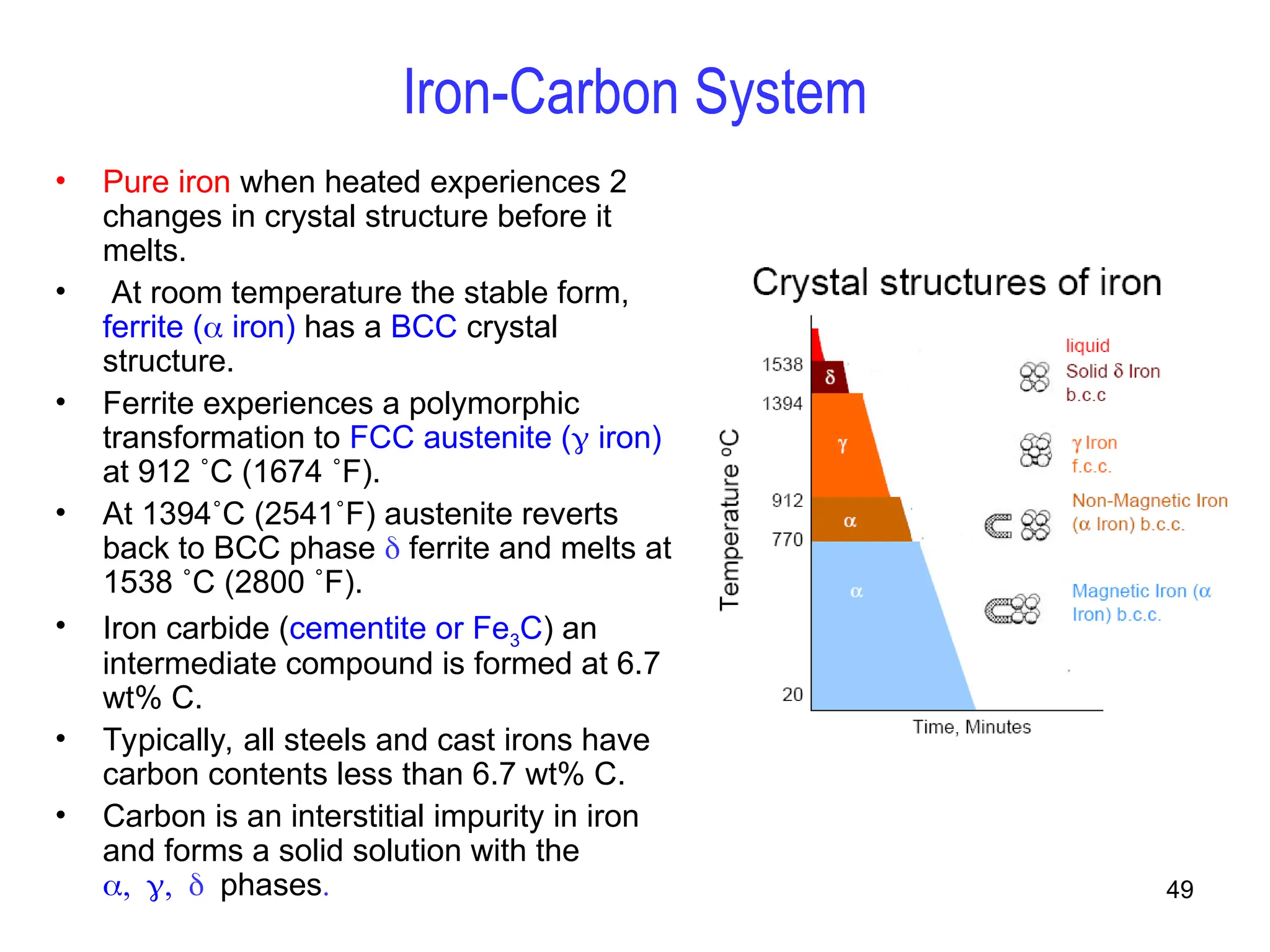
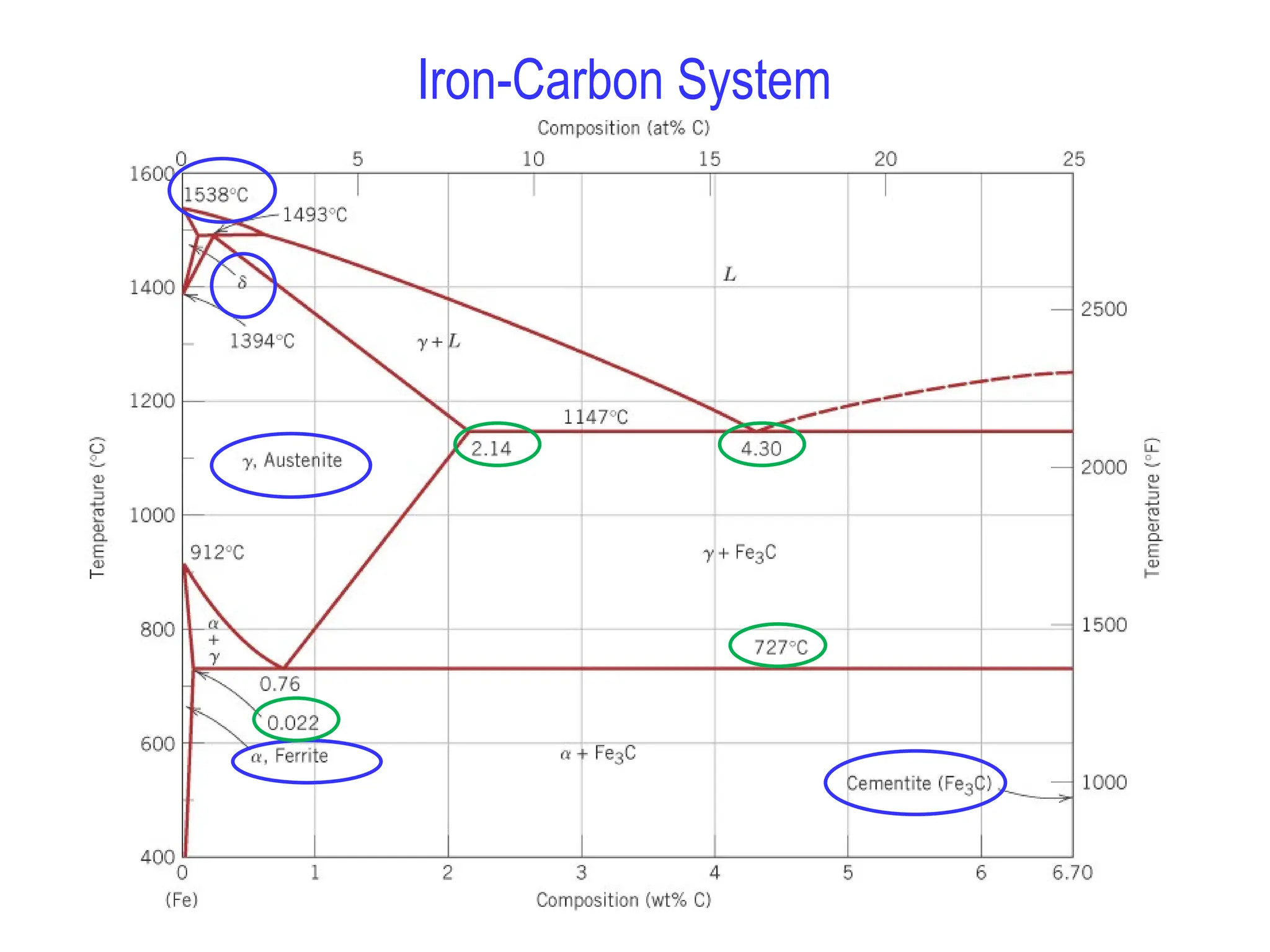
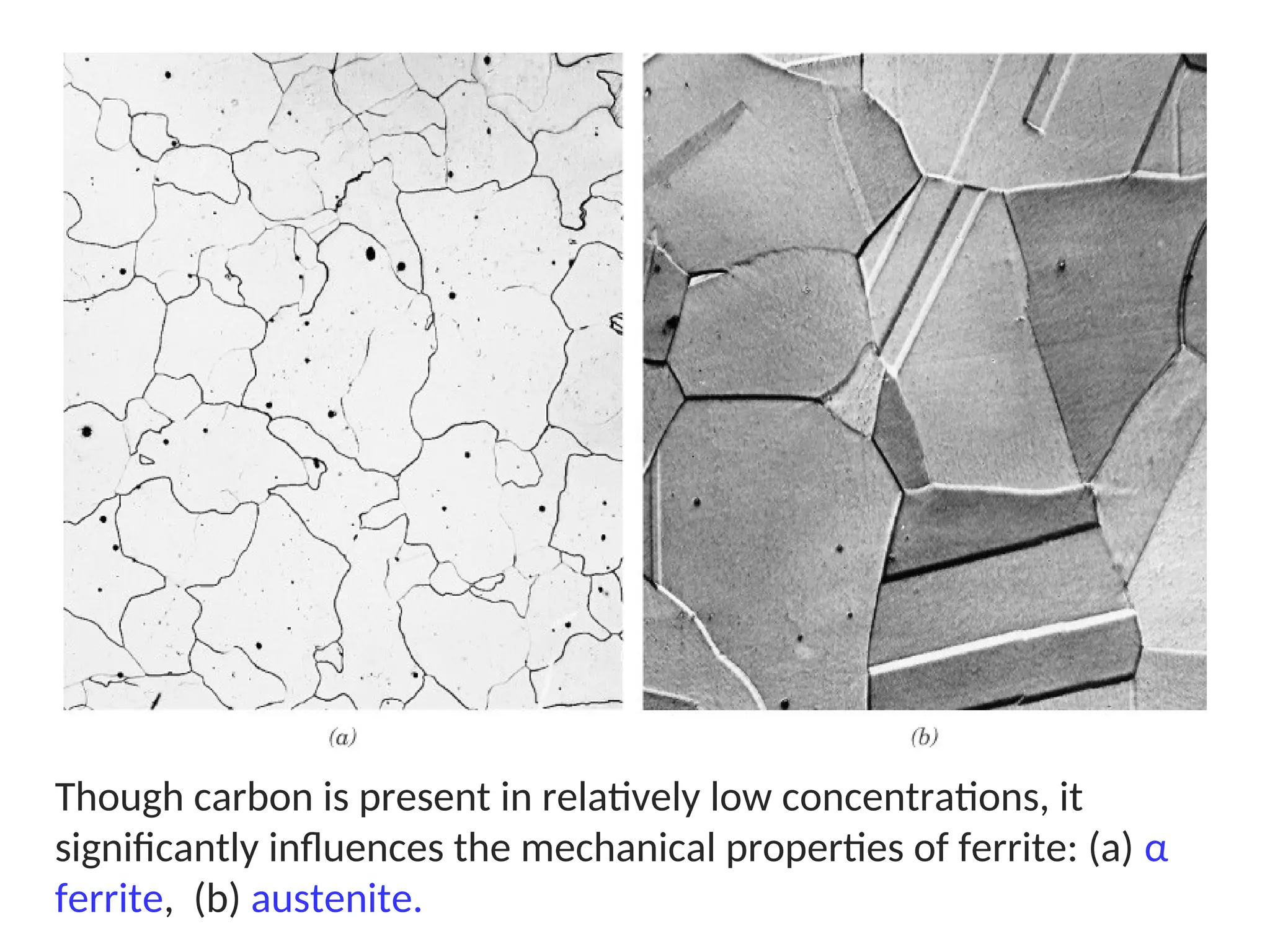
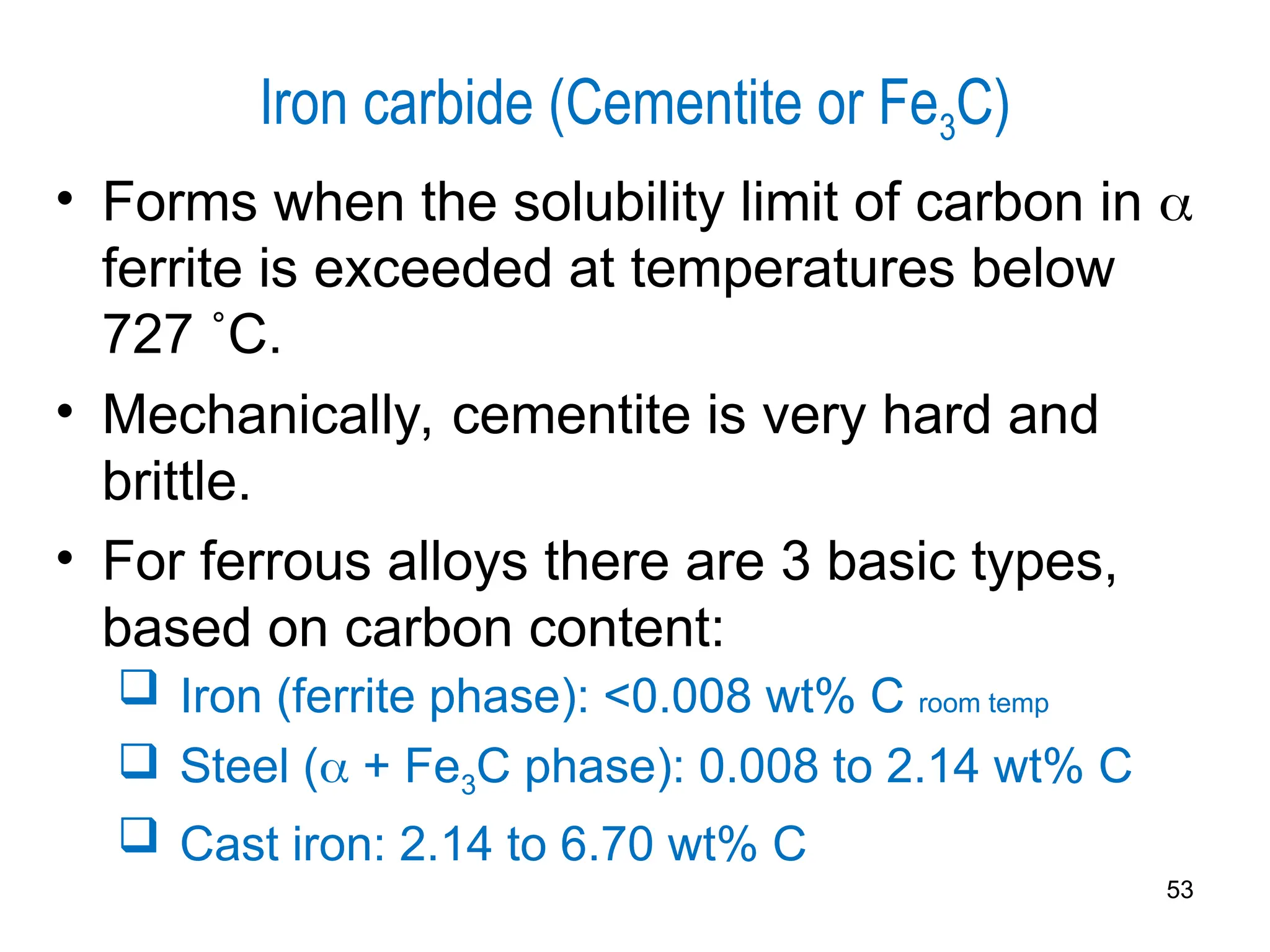
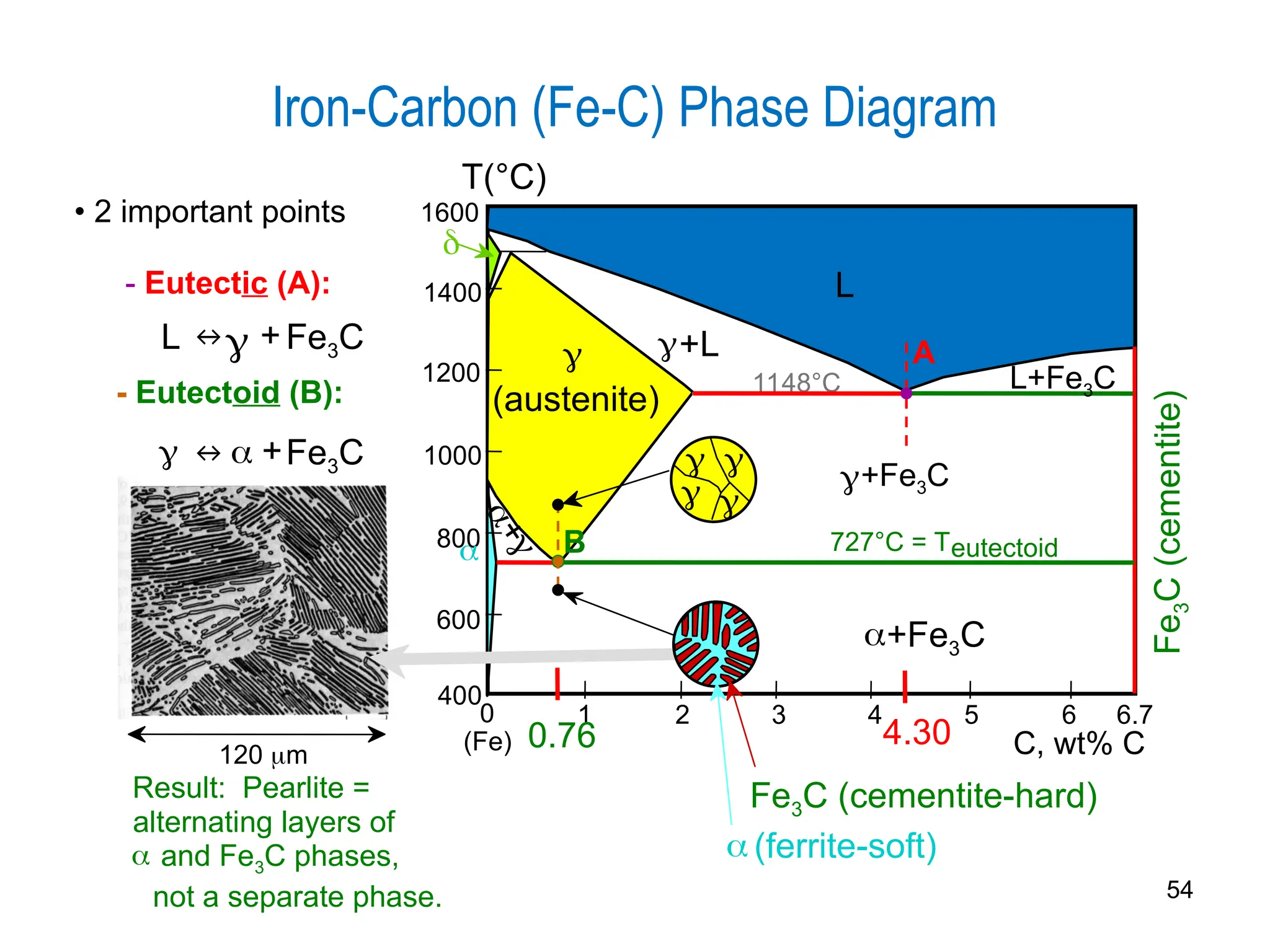
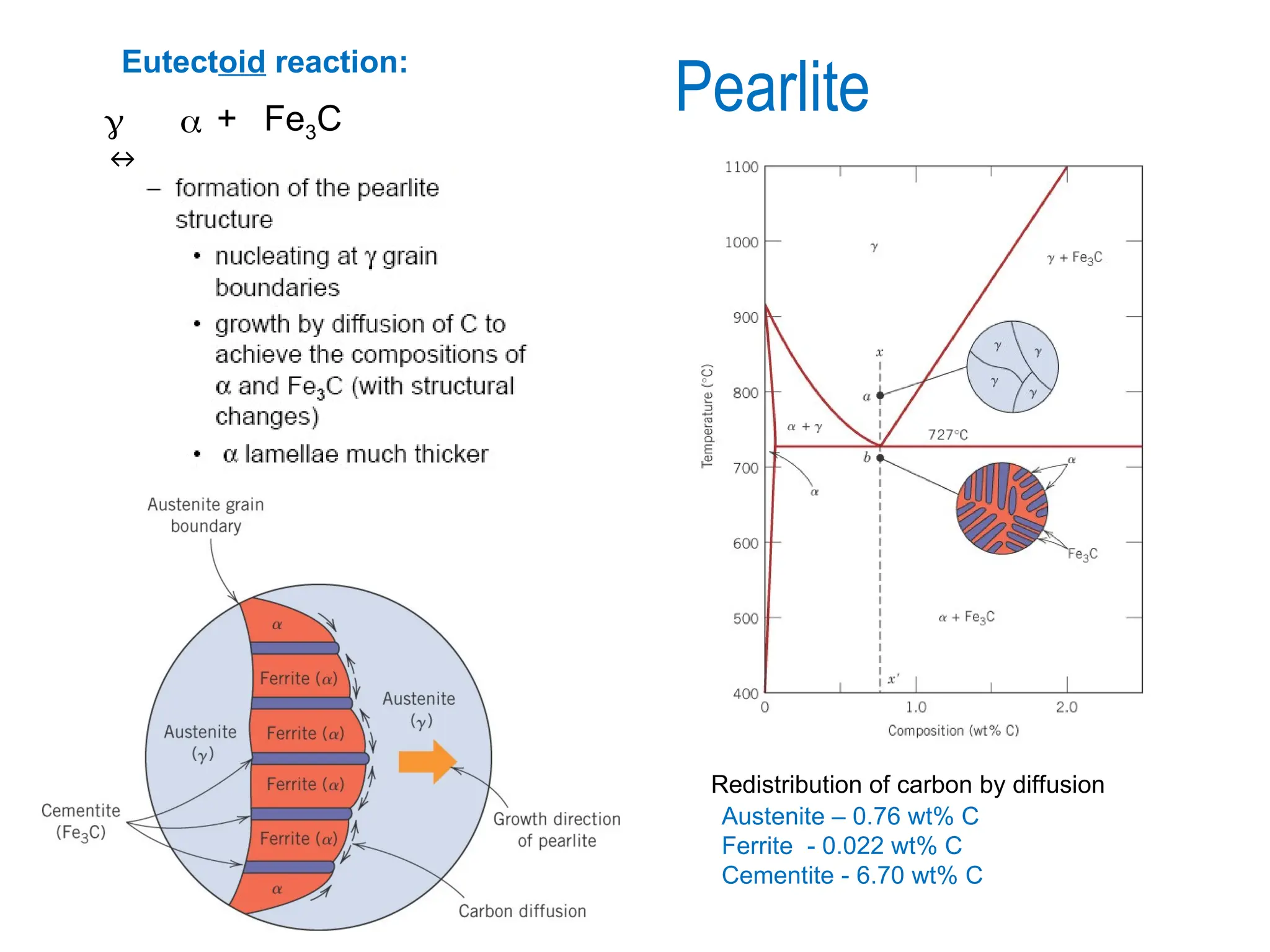
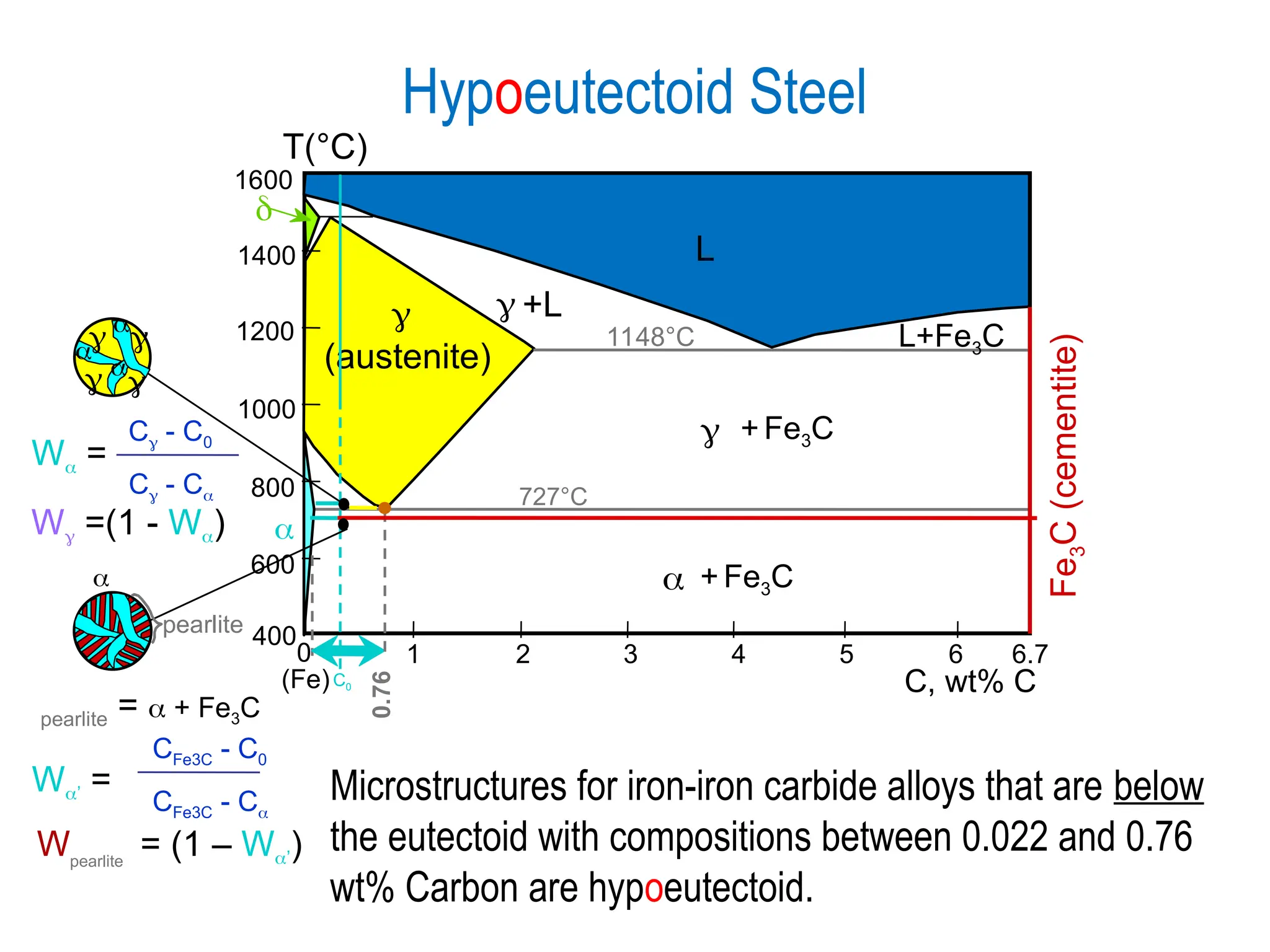
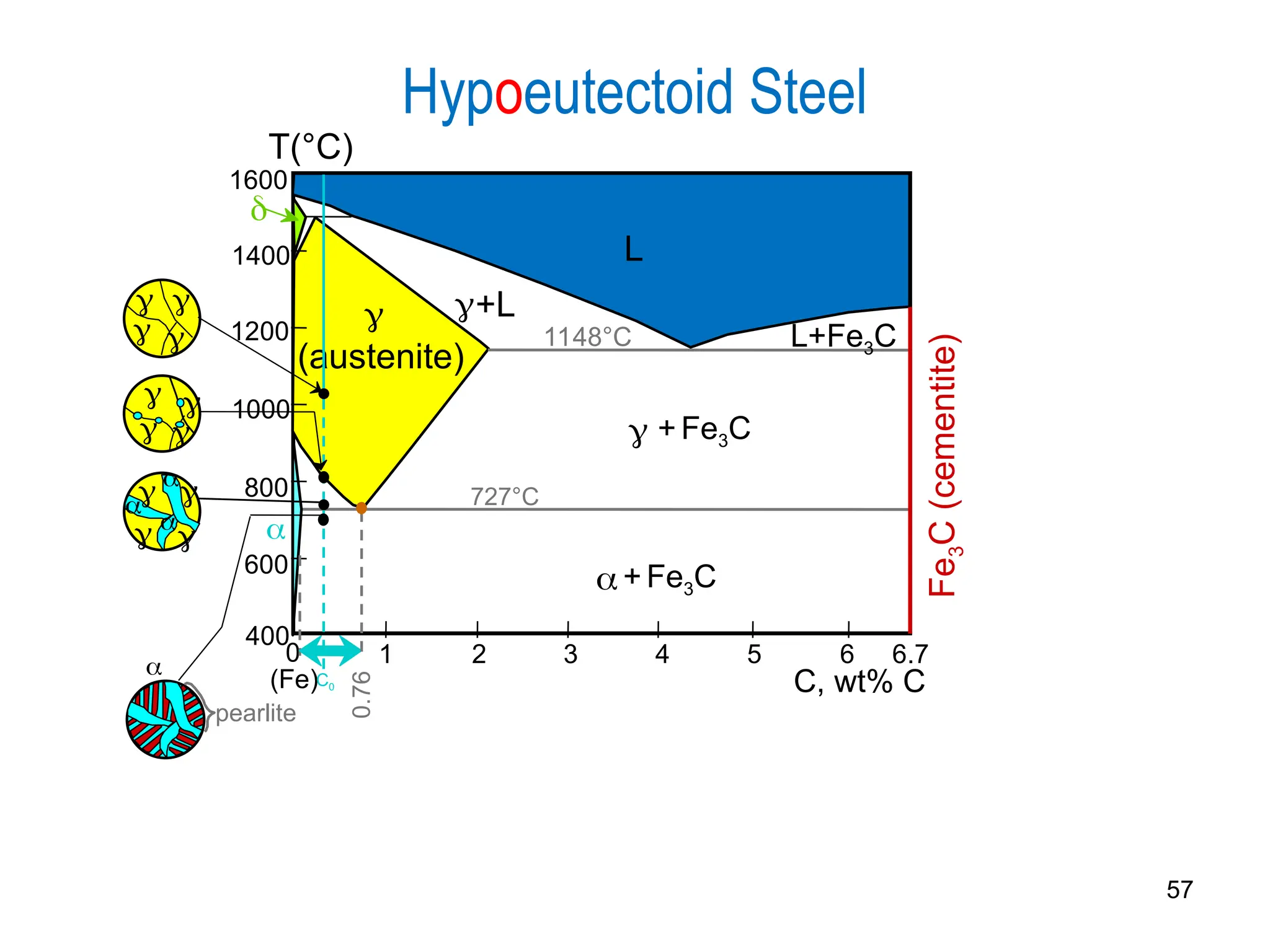
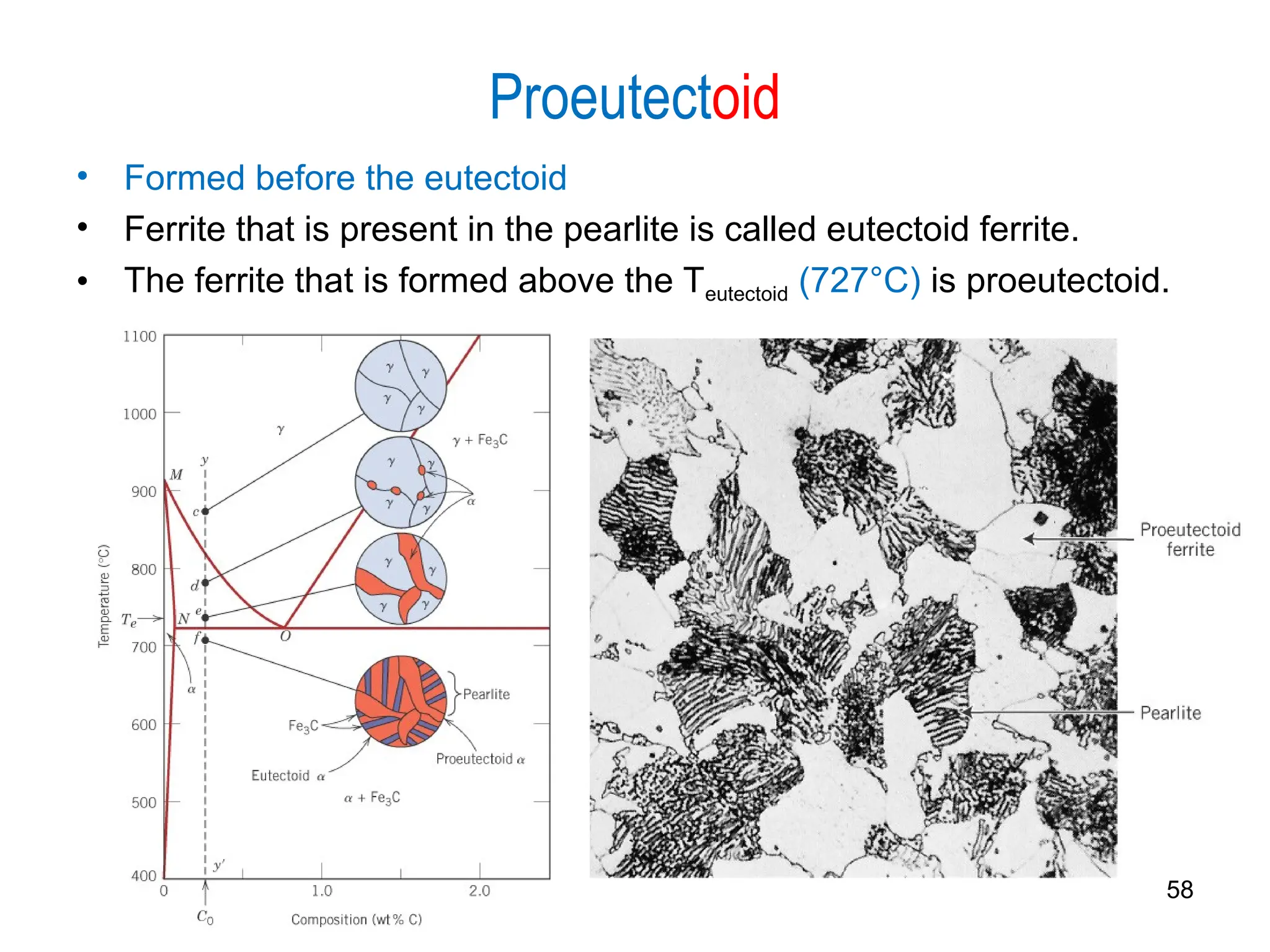
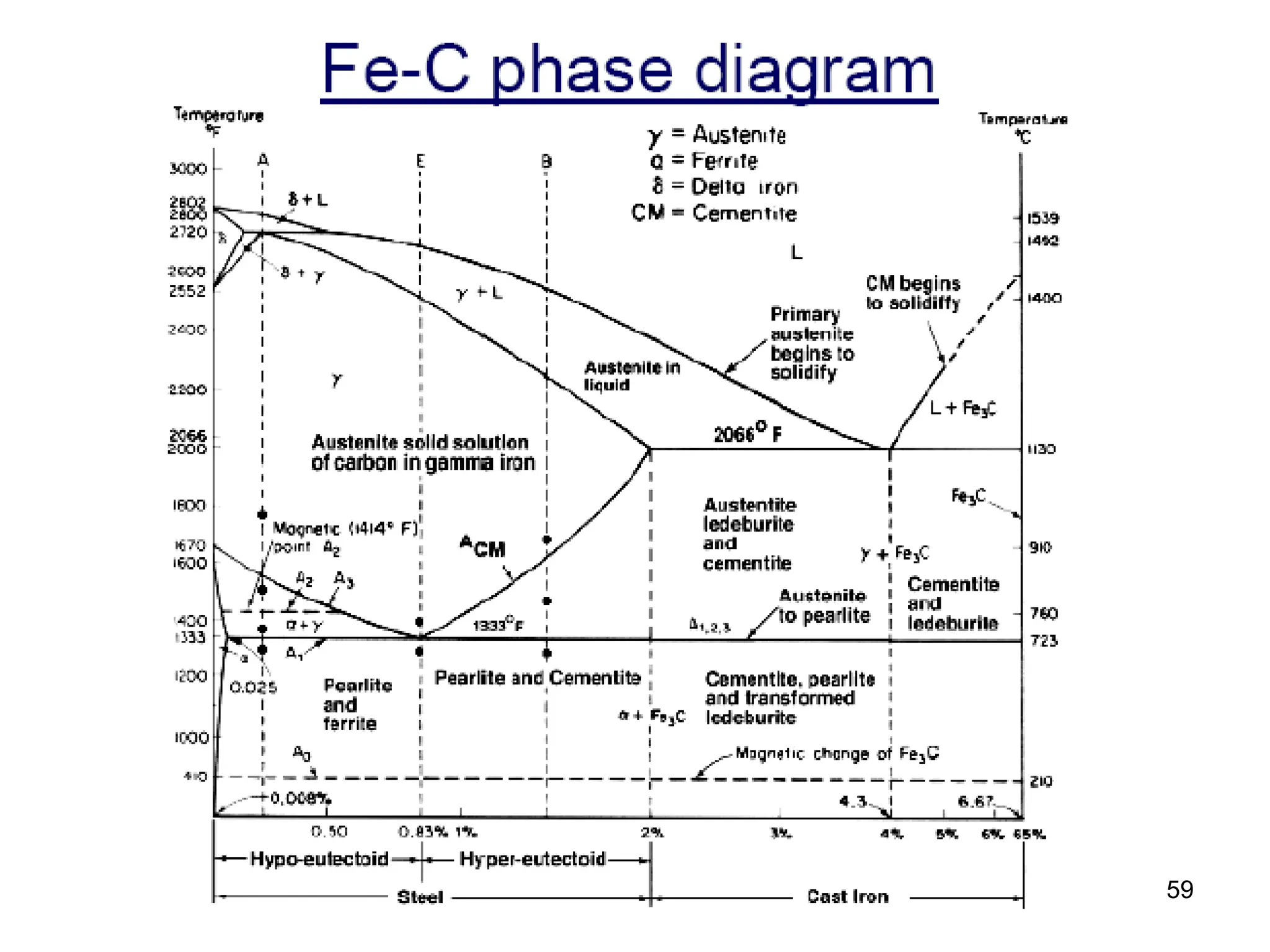
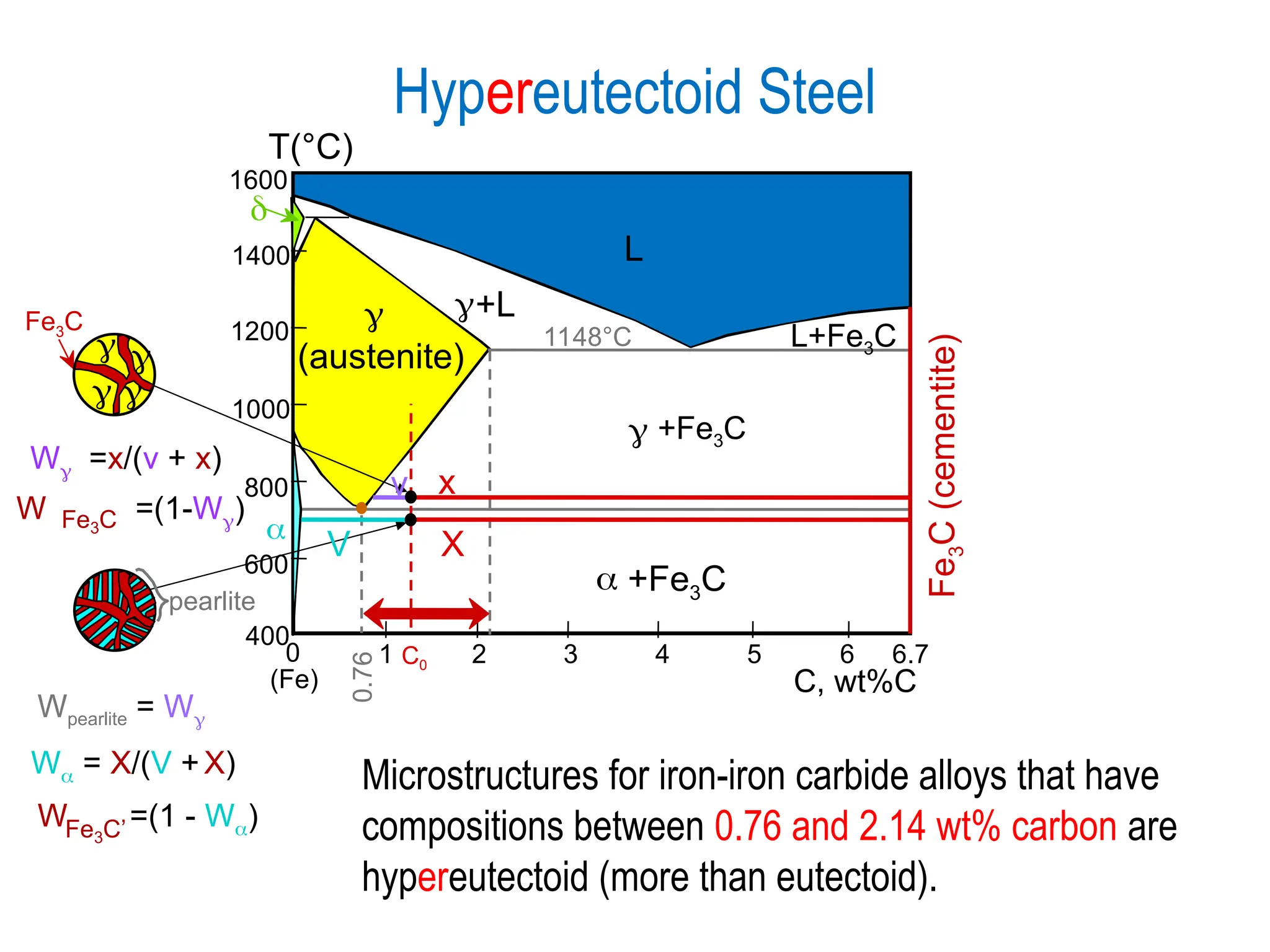
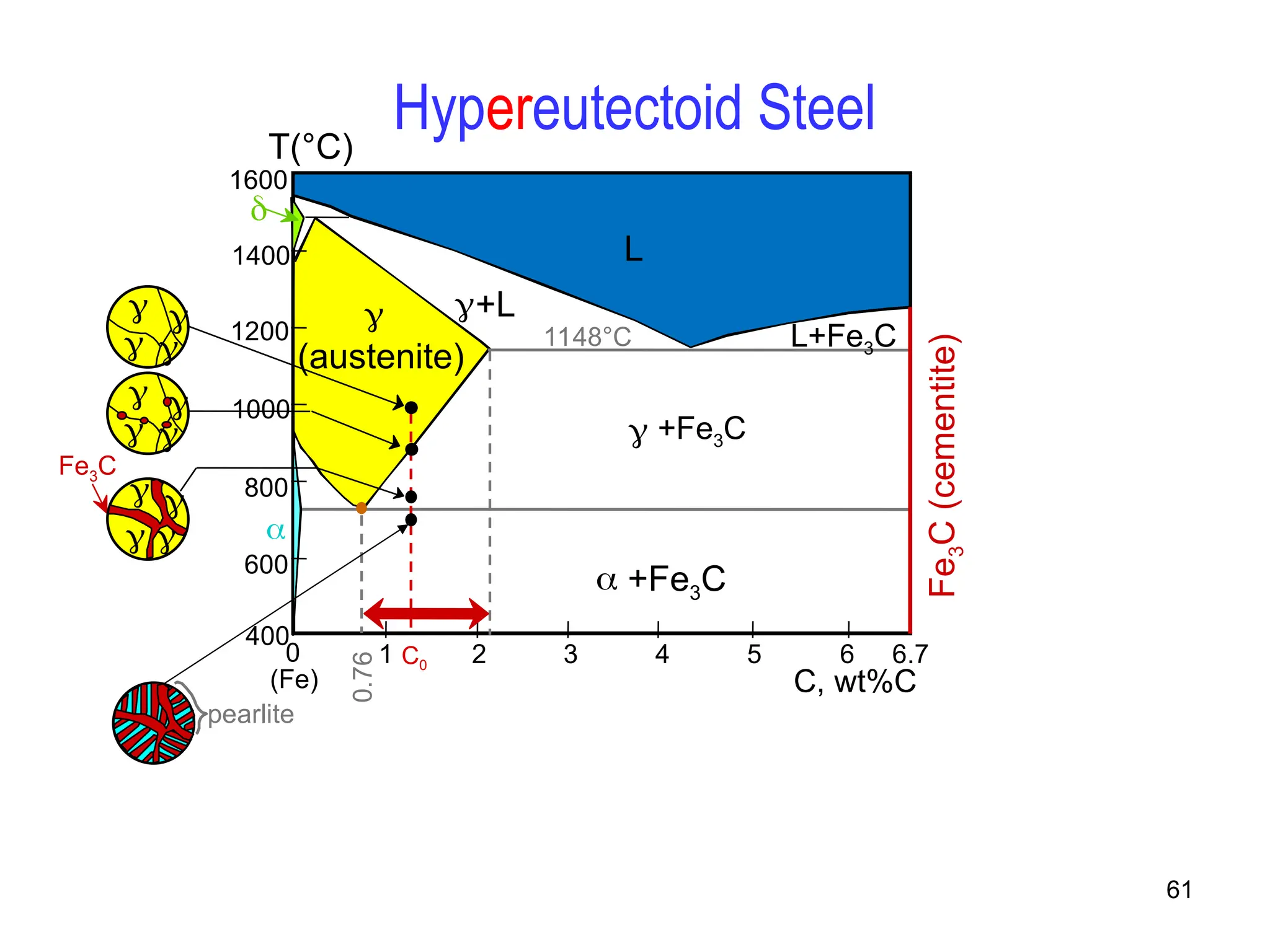
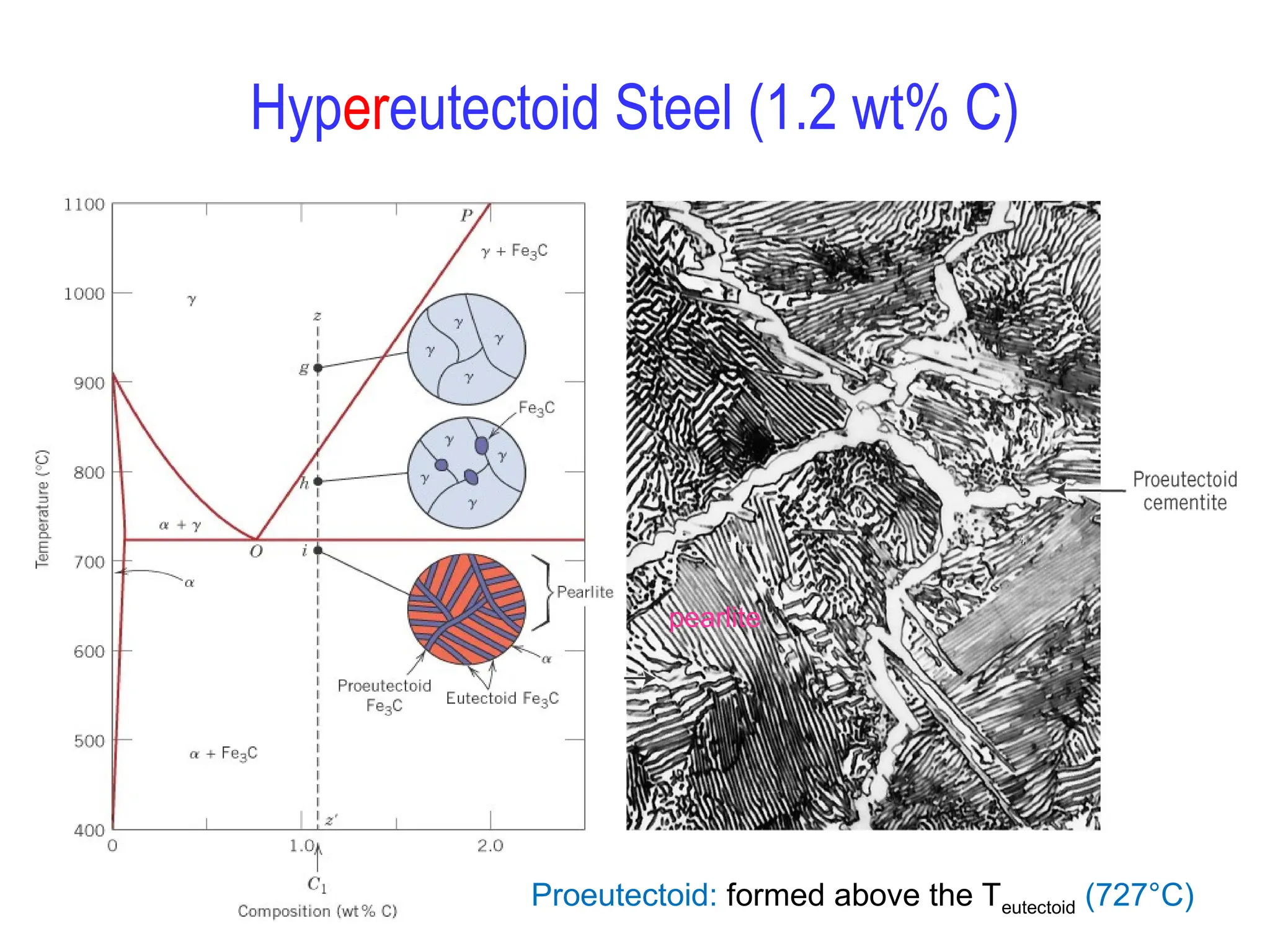
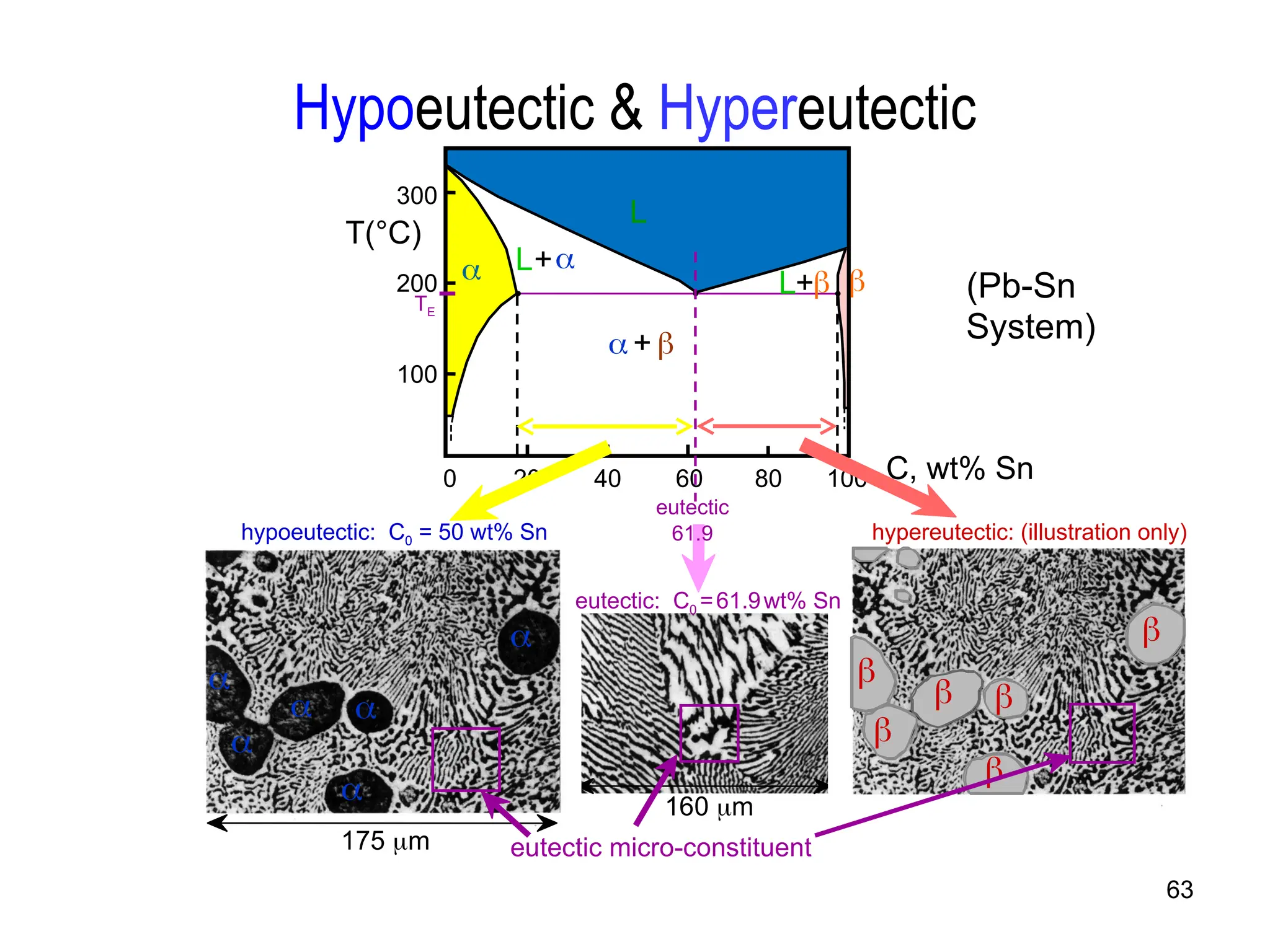
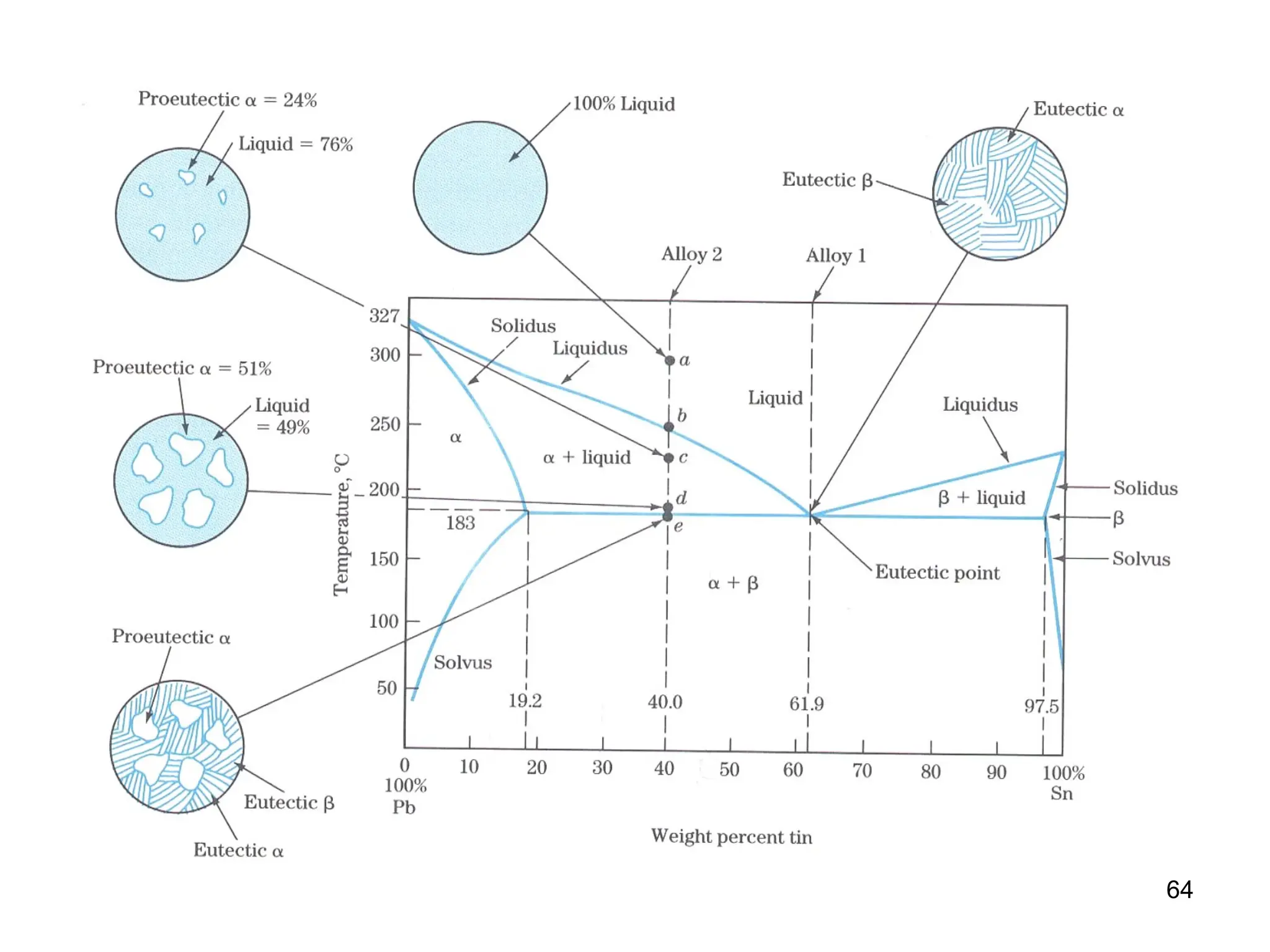
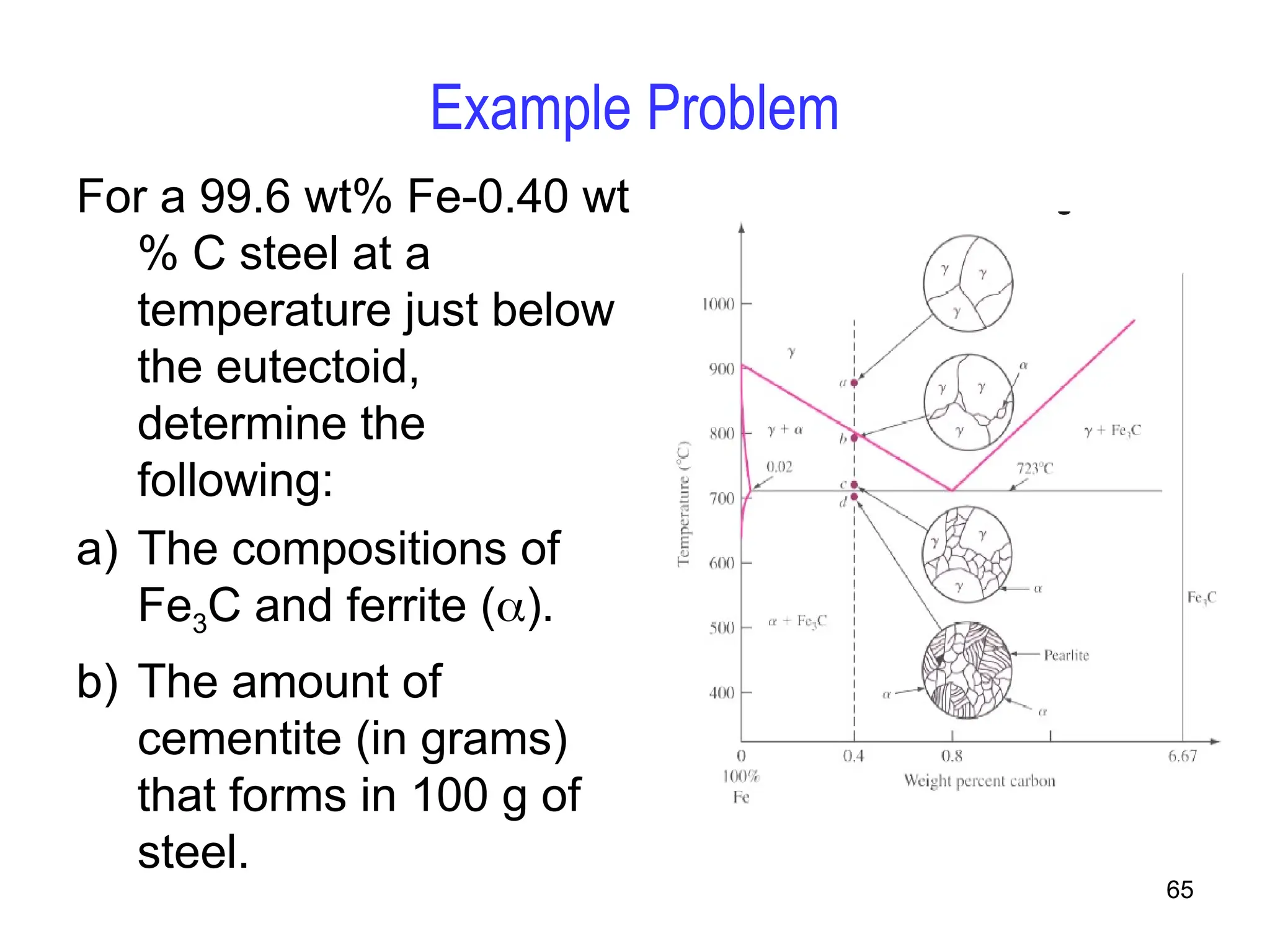
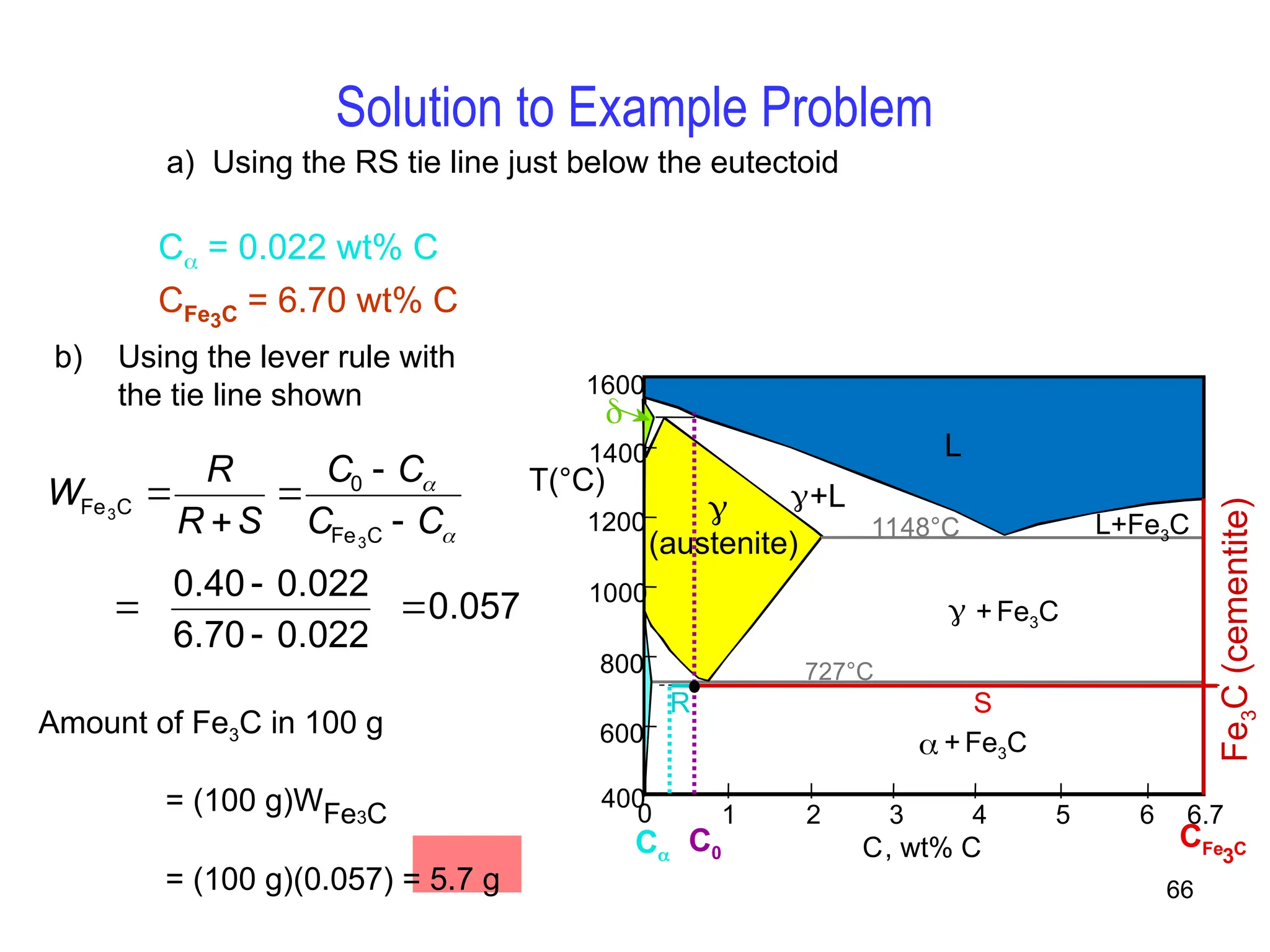
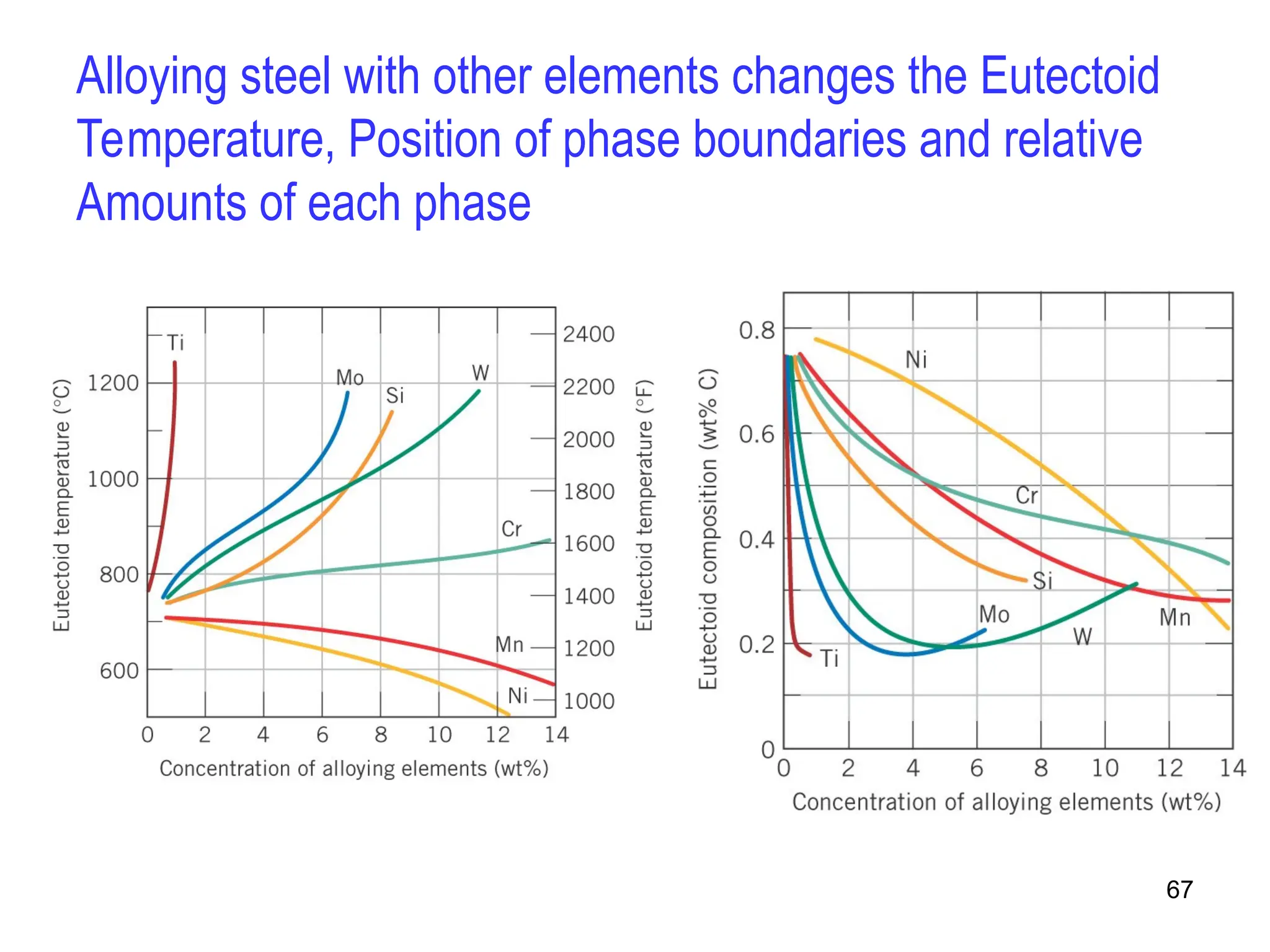
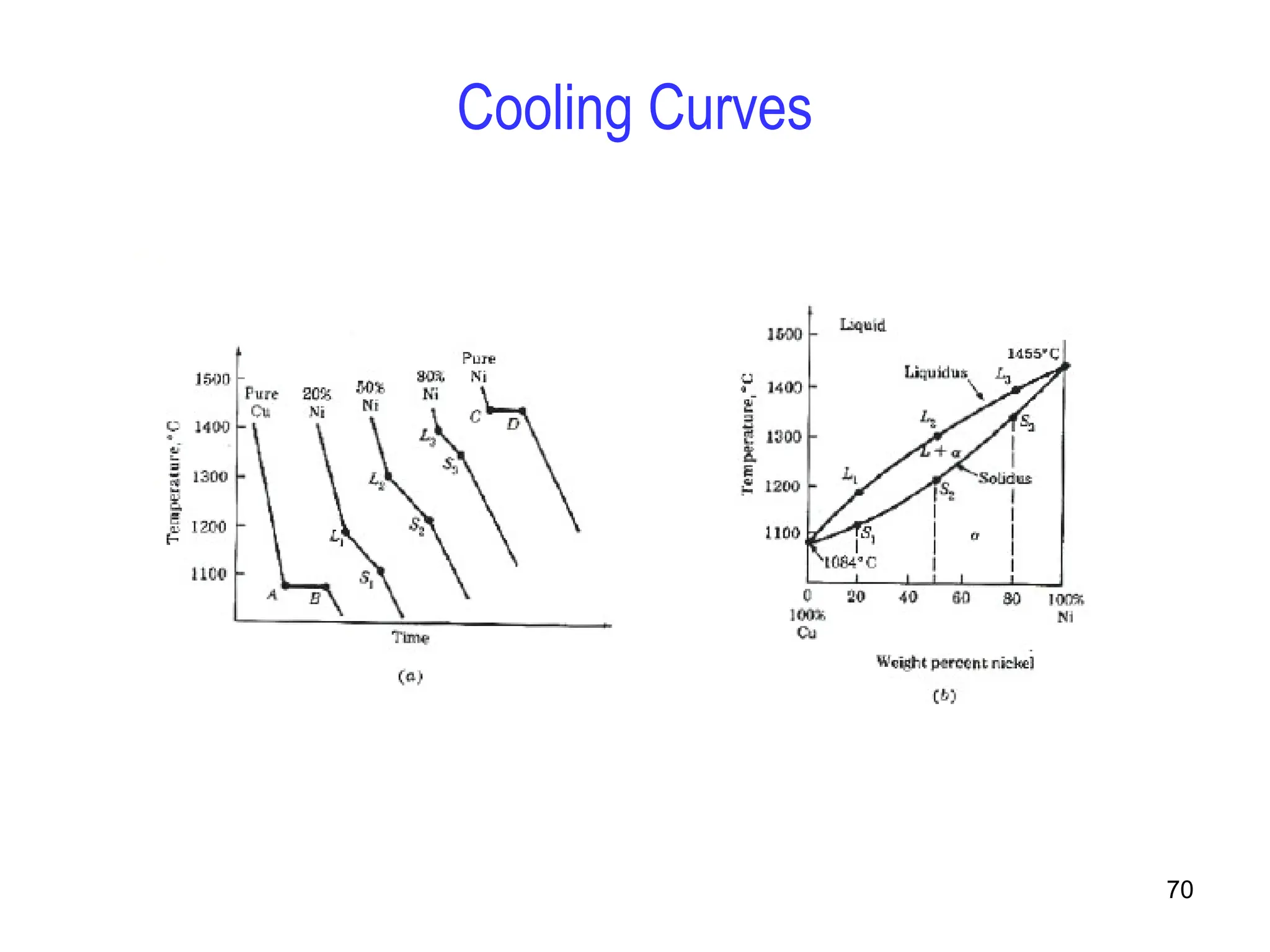
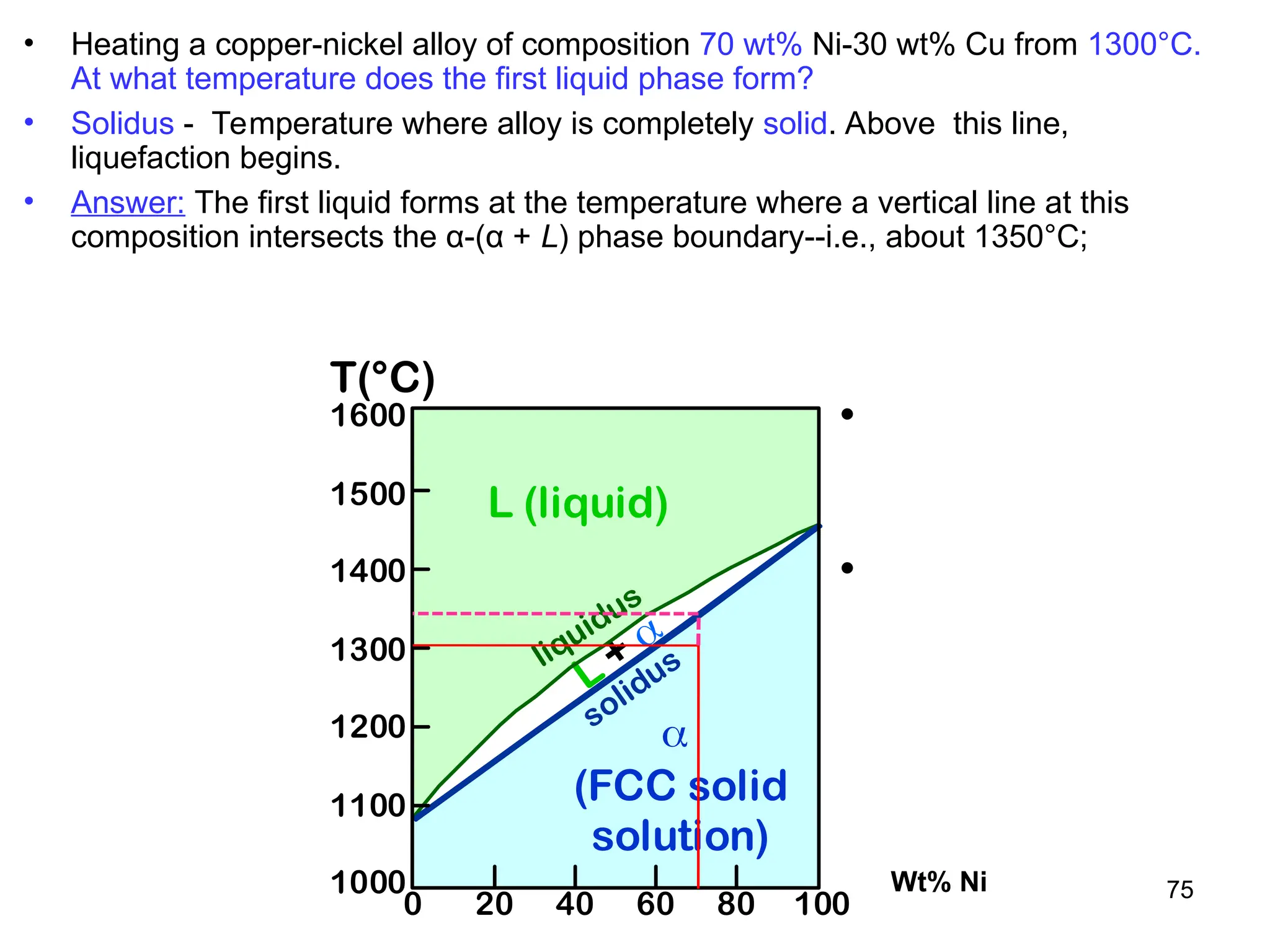
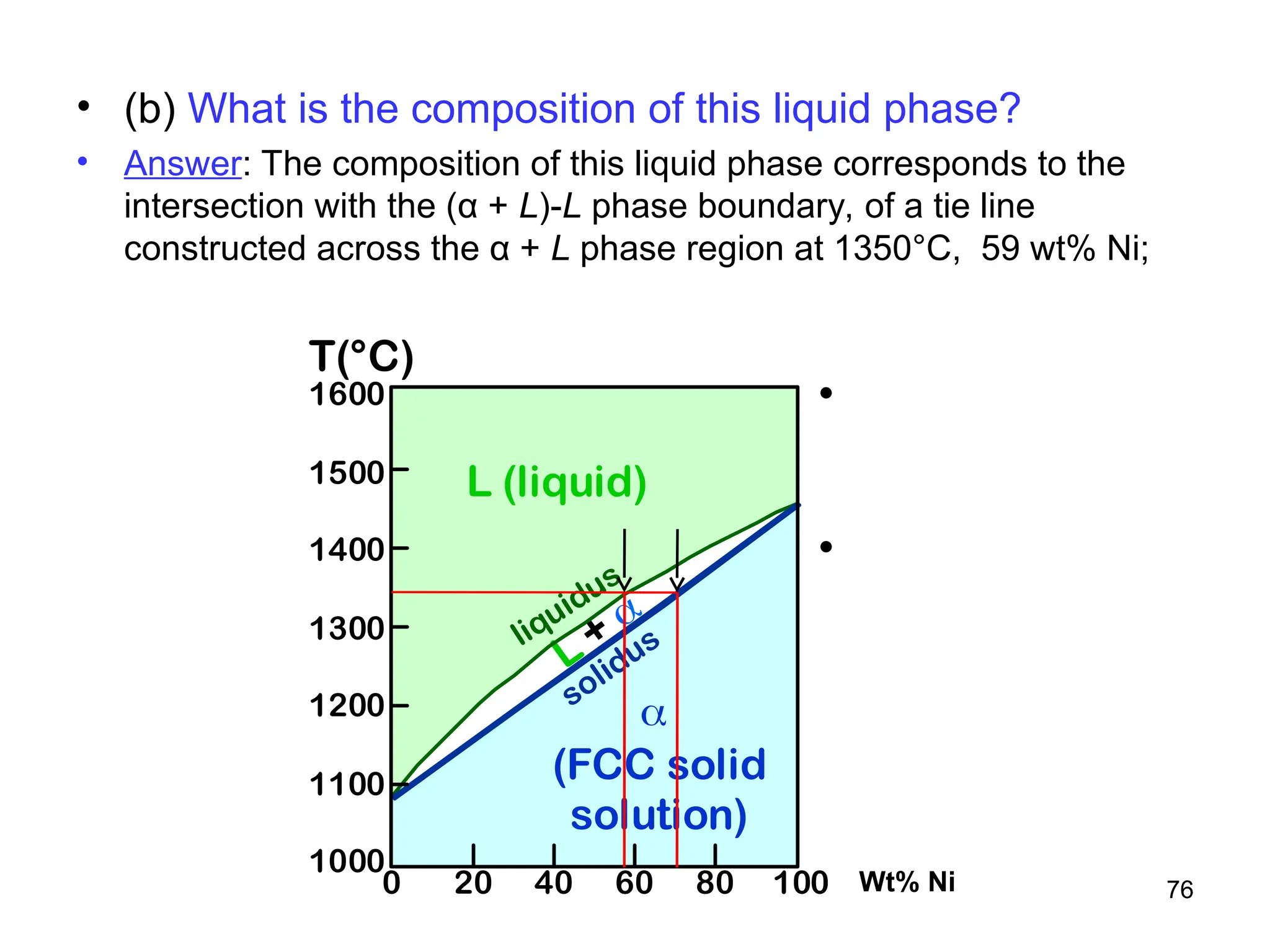
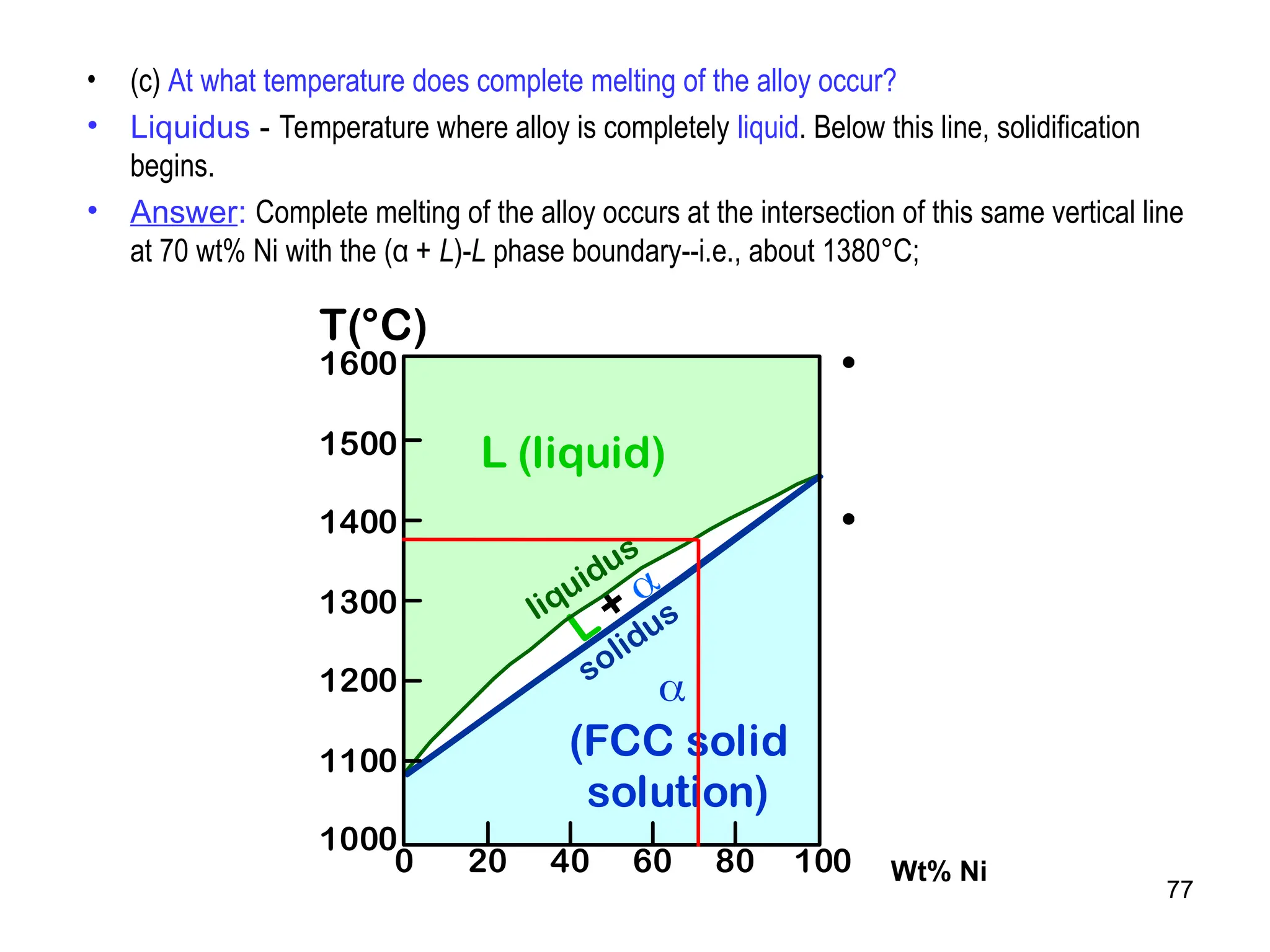
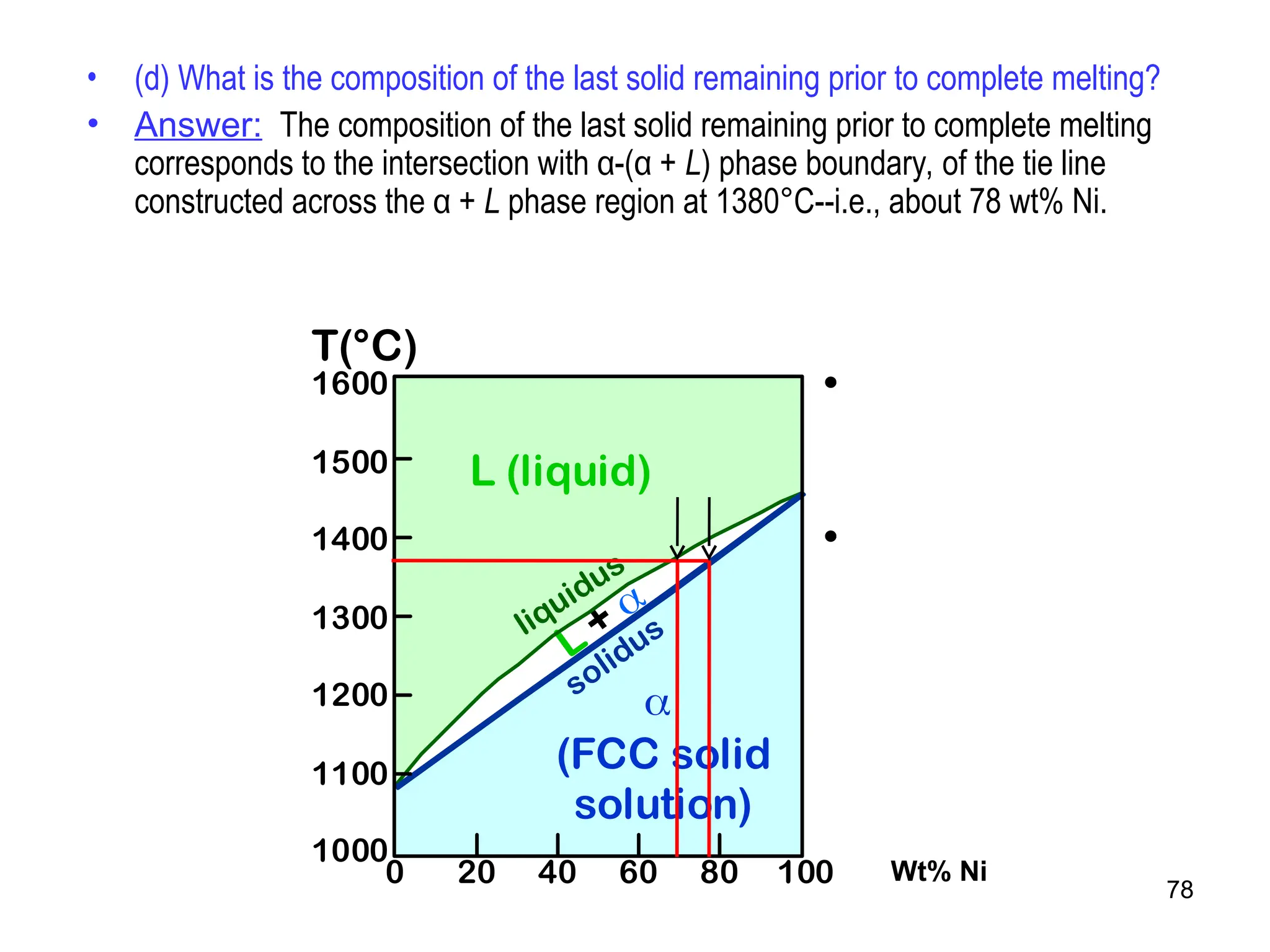
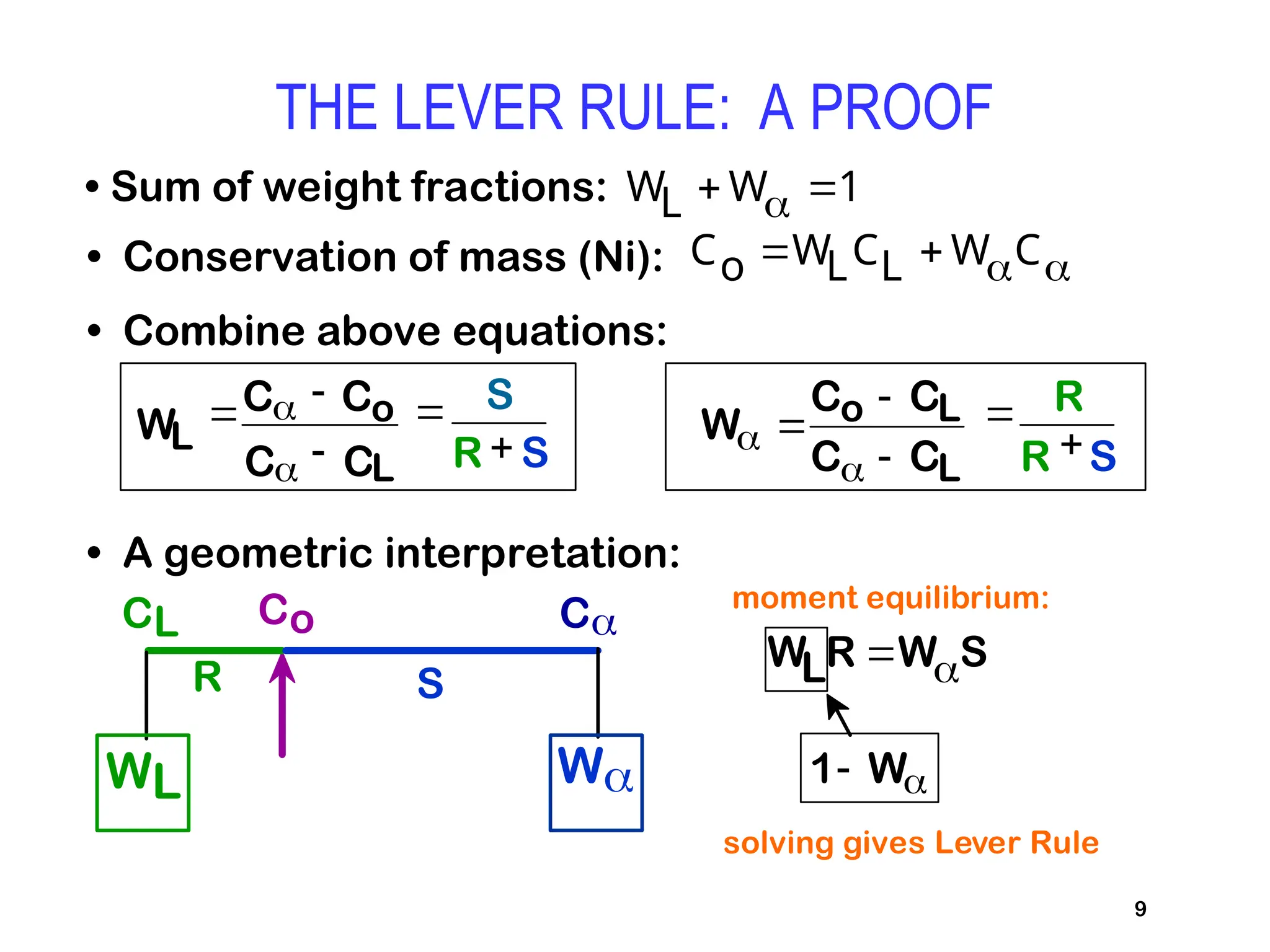
Chapter 10 discusses phase diagrams focusing on solubility limits, phases, and phase equilibria in binary alloy systems, particularly using copper-nickel and copper-silver as examples. It explains the concepts of one-component and two-component systems, their phase behaviors under varying temperature and composition, and the implications for microstructure and mechanical properties of alloys. The chapter emphasizes the importance of phase diagrams in understanding material behavior during processes like melting, casting, and solidification.