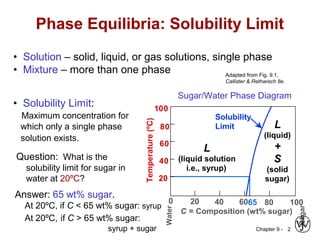
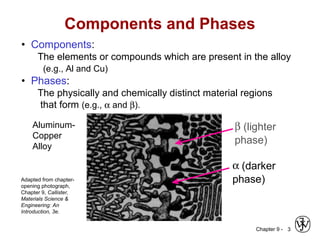
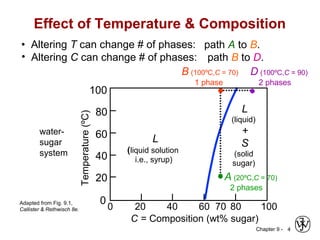
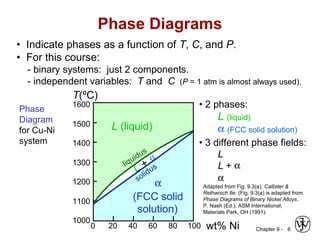
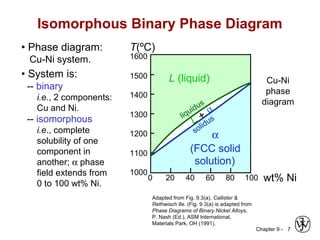
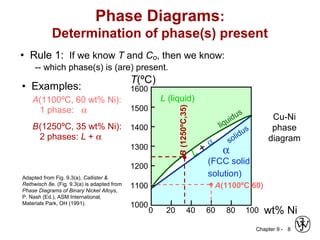
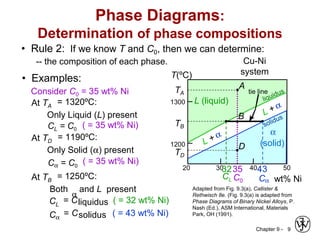
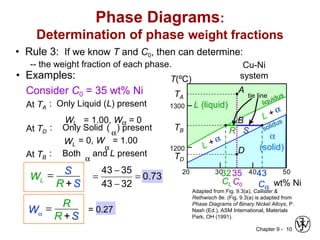
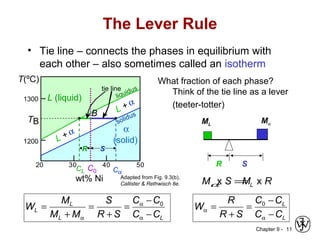
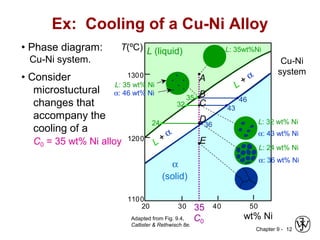
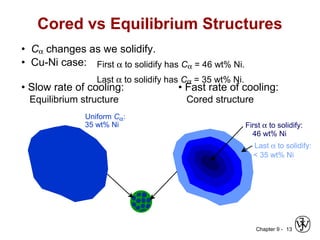
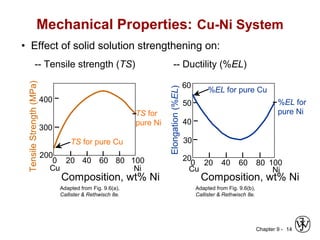
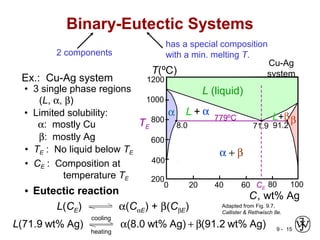
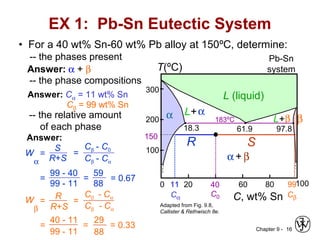
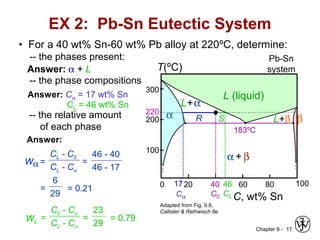
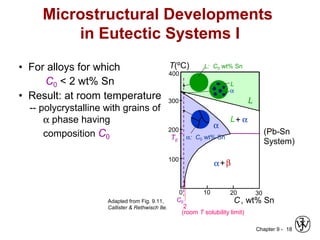
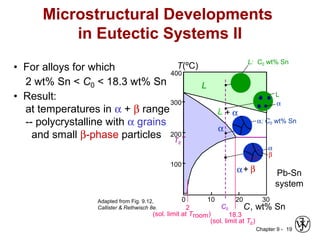
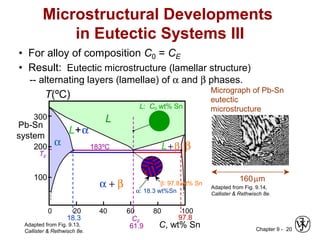
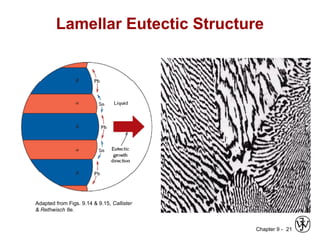
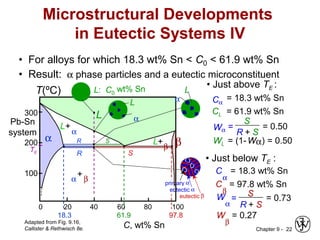
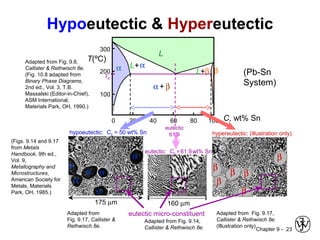
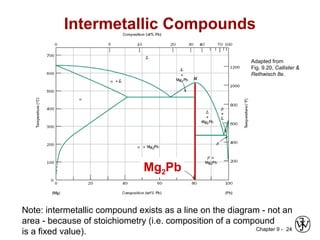
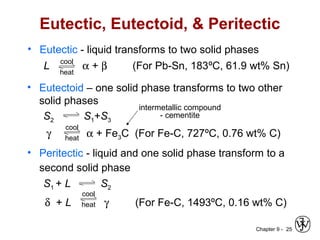
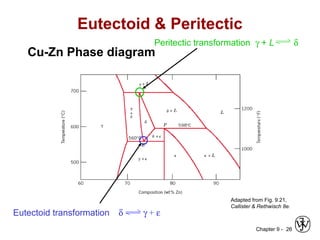
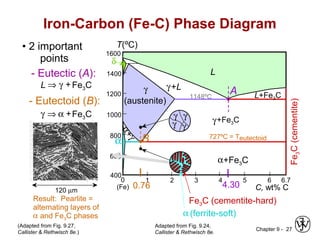
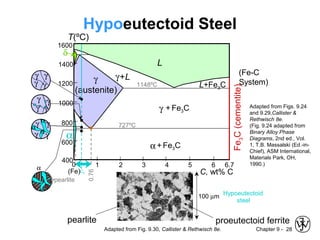
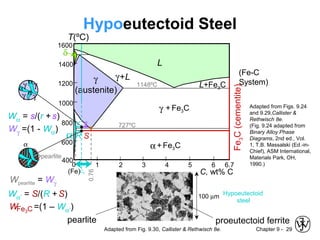
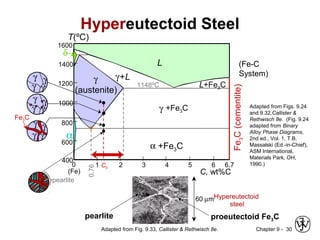
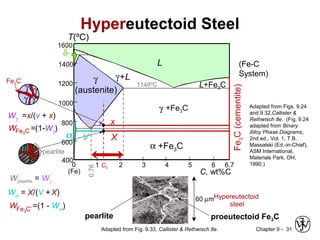
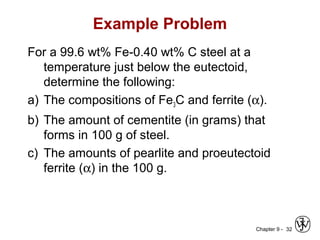
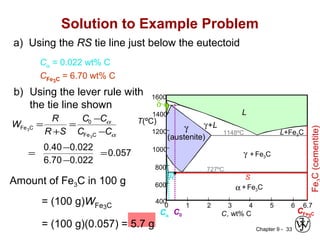
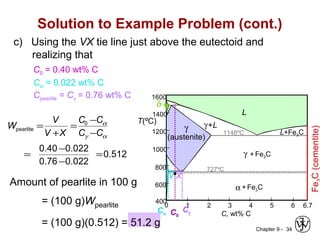
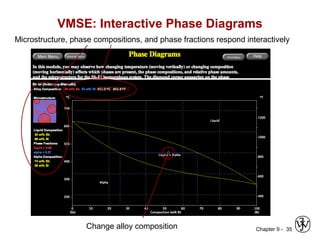
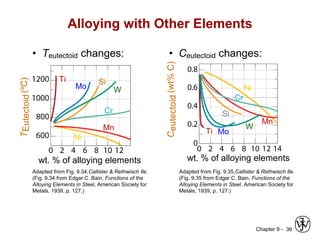
This document discusses phase diagrams and microstructures in binary alloy systems. It begins by outlining key questions that can be addressed using phase diagrams, such as the phases present at a given temperature and composition. It then provides examples of using a Cu-Ni phase diagram to determine phase compositions and fractions. The document discusses eutectic systems and uses a Pb-Sn phase diagram to solve examples determining phase properties. It concludes by describing microstructures that form in the Pb-Sn system depending on alloy composition.