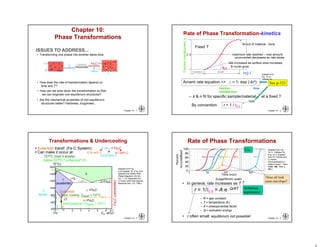
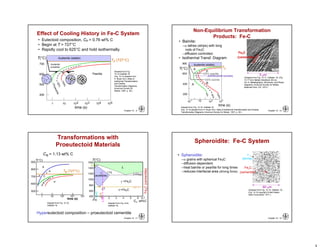
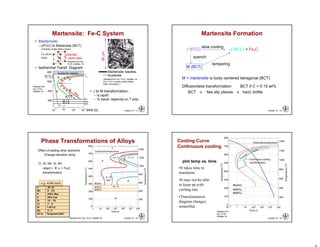
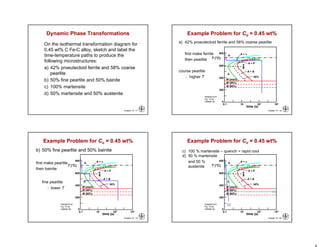
This chapter discusses phase transformations in materials. It addresses key issues like how the rate of transformation depends on time and temperature. Non-equilibrium structures can be engineered by slowing down transformations. The chapter examines the kinetics of phase transformations using models like the Avrami equation. It explores transformations in various material systems like iron-carbon alloys and how processing conditions like cooling rates affect microstructure and properties.