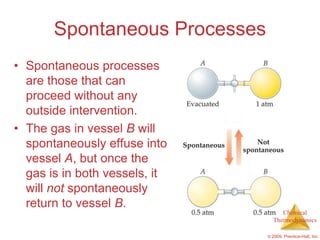
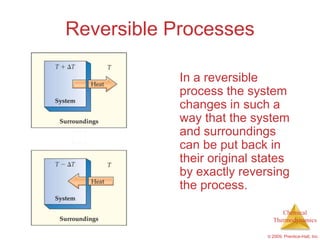
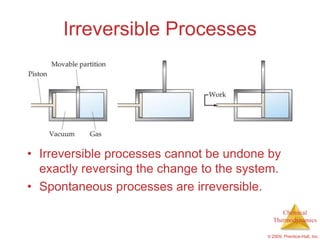
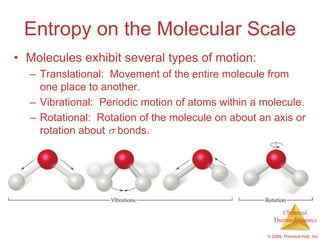
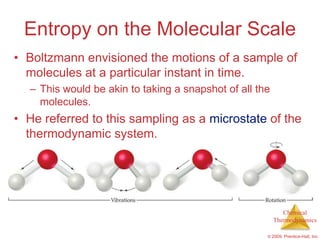
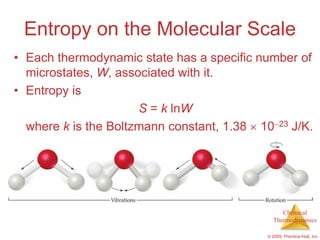
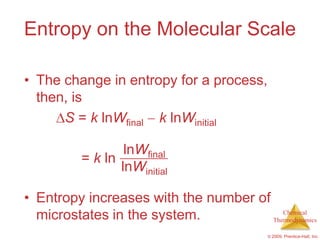
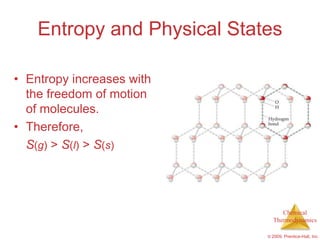
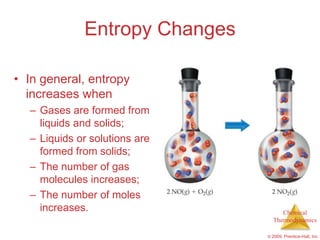
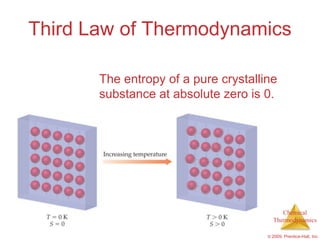
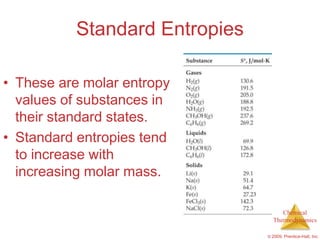
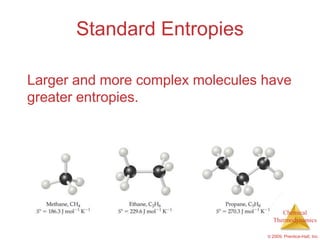
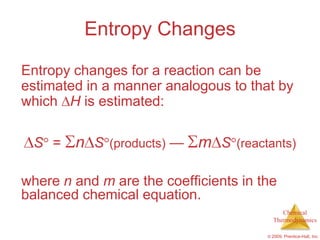
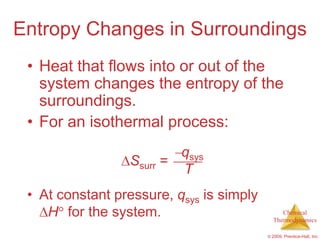
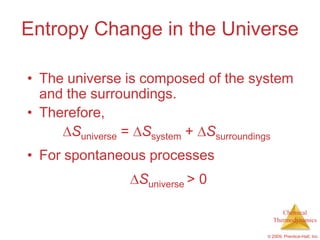
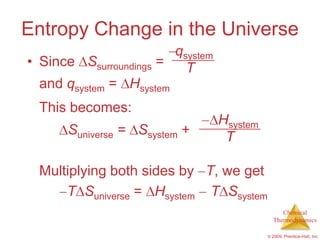
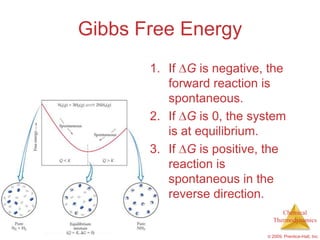
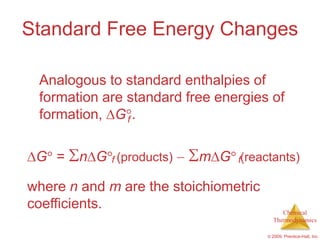
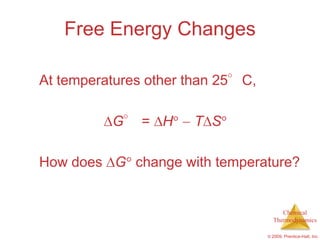
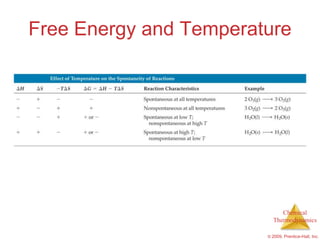
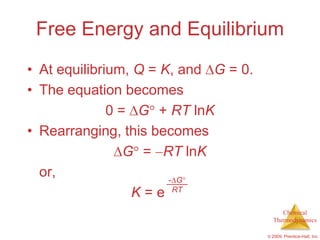
This document summarizes key concepts from Chapter 19 of Chemistry, The Central Science. It discusses the first and second laws of thermodynamics, including that energy is conserved but entropy increases for spontaneous processes. It also describes entropy on the molecular scale in terms of microstates and how entropy increases with disorder. Finally, it introduces Gibbs free energy and how a negative value indicates a spontaneous reaction at equilibrium.