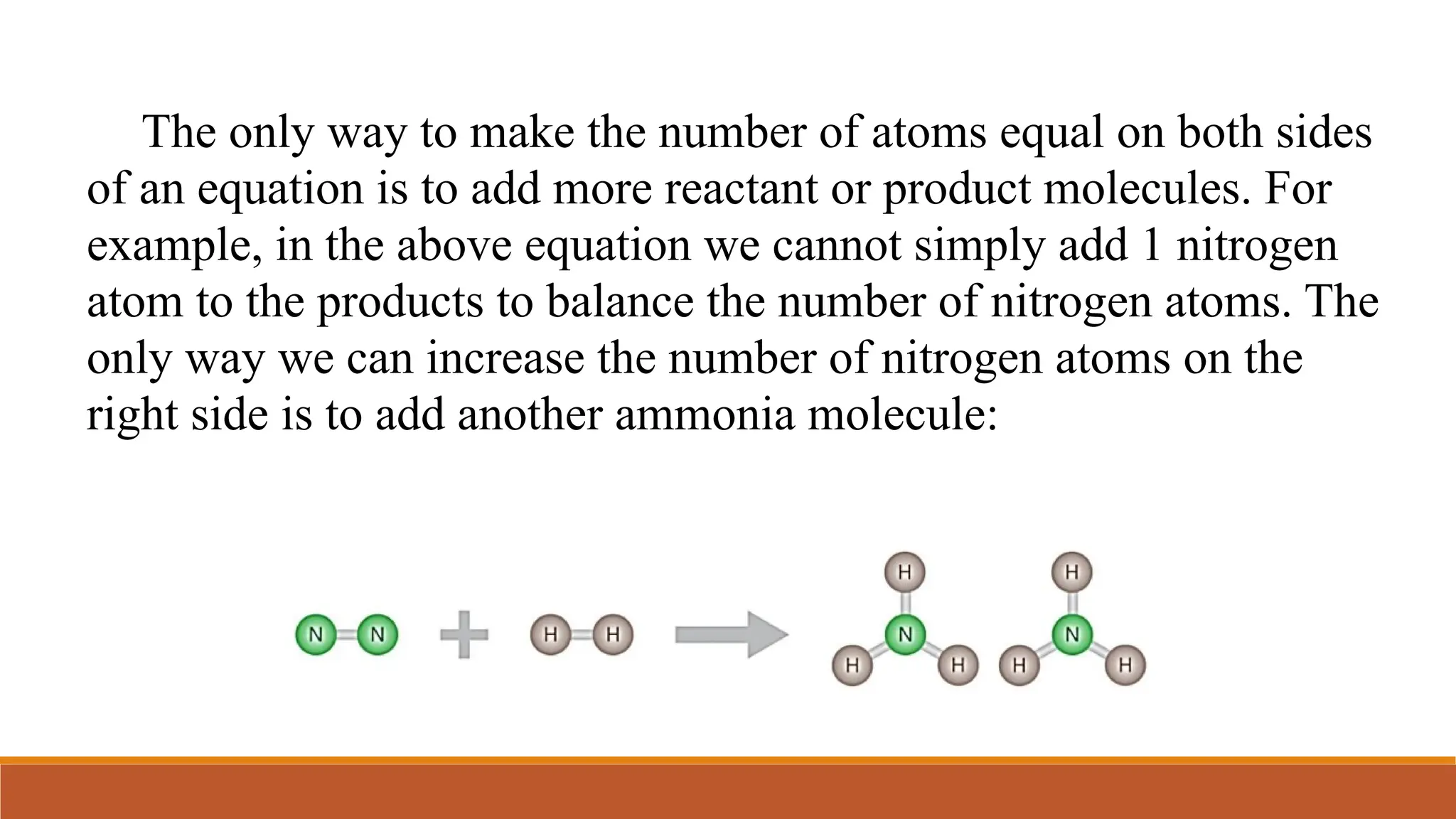
This document outlines key concepts related to mass relationships in chemical reactions, including calculation of molecular formulas, balancing equations, and determining mole or mass ratios. It provides various examples and problems that cover topics such as limiting reagents, percent yield, and stoichiometry. The importance of the law of conservation of mass and the methods for representing chemical reactions through equations are also discussed.