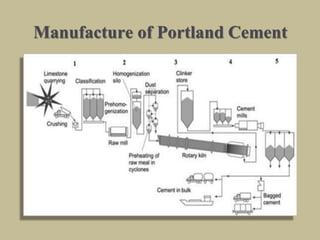
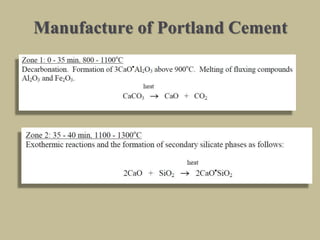
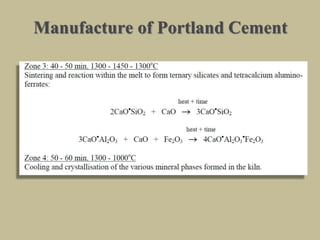
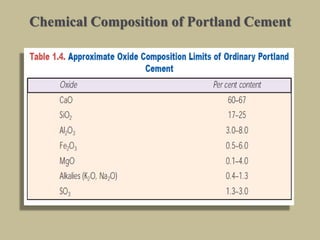
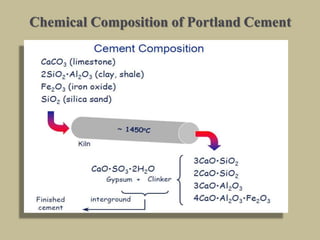
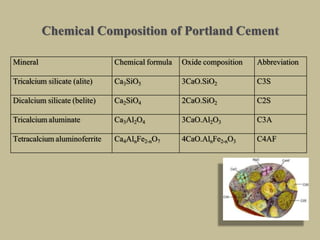
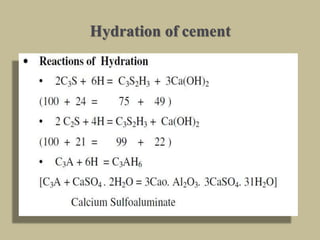
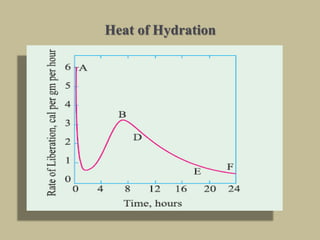
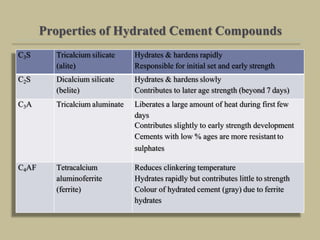
Cement is produced by heating limestone and clay at high temperatures to form clinker, which is then ground with gypsum. The key compounds formed are tricalcium silicate, dicalcium silicate, tricalcium aluminate, and tetracalcium aluminoferrite. When mixed with water, cement undergoes hydration reactions that cause it to harden over time. Tricalcium silicate reacts rapidly and contributes to early strength, while dicalcium silicate reacts slowly and provides later strength. Tricalcium aluminate also reacts quickly but is retarded by gypsum addition. The reactions are exothermic and generate heat.