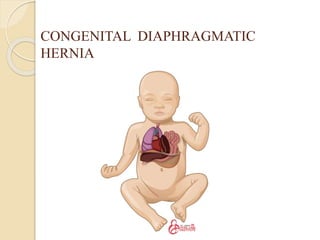
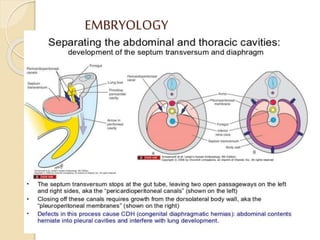
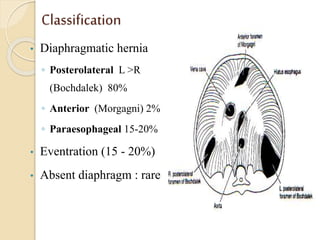
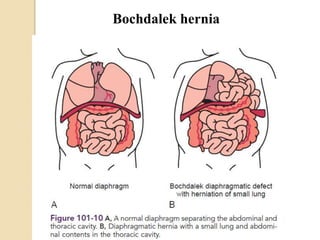
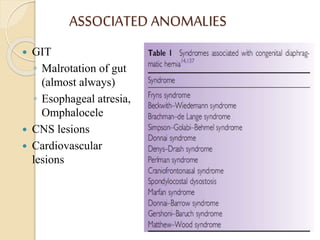
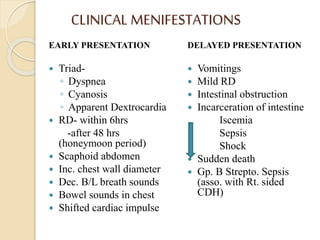
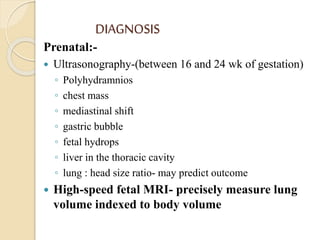
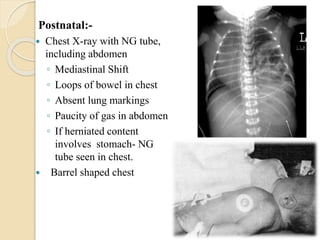
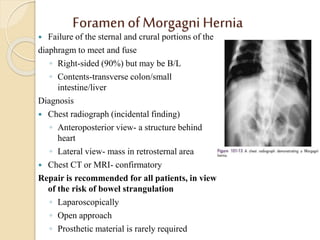
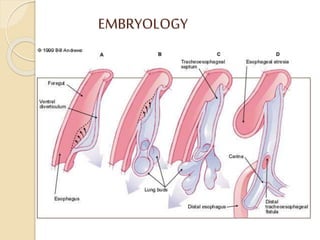
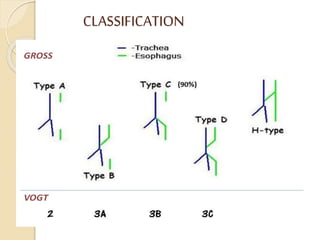
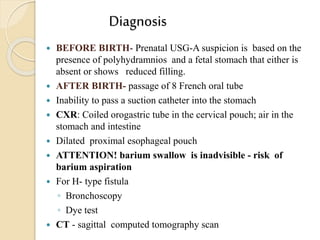
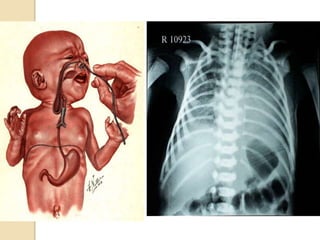
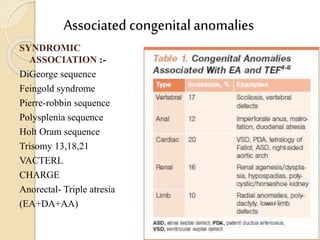
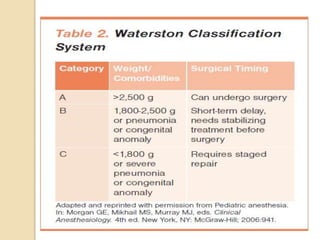
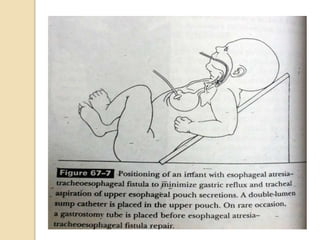
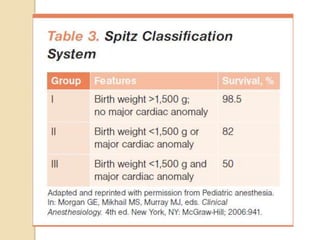
This document discusses congenital diaphragmatic hernia and tracheoesophageal fistula. It begins by defining congenital diaphragmatic hernia and describing the different types. It then discusses the history, embryology, pathophysiology, classification, clinical manifestations, diagnosis, treatment including surgical repair, complications, and prognosis of congenital diaphragmatic hernia. It also defines tracheoesophageal fistula, describes the different types, discusses embryology, pathophysiology, clinical presentation, diagnosis, associated anomalies, preoperative preparation, surgical repair techniques, and complications.