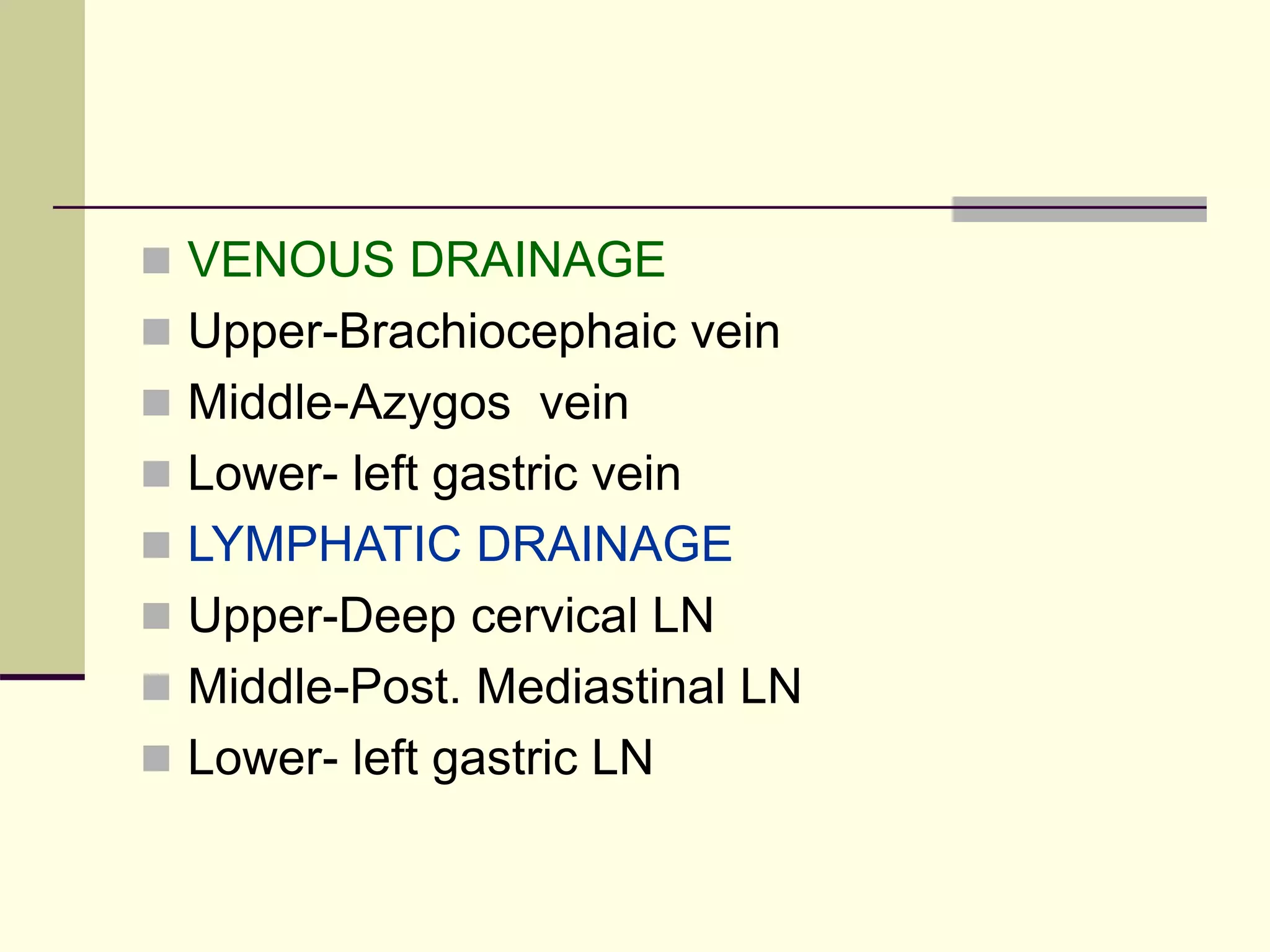
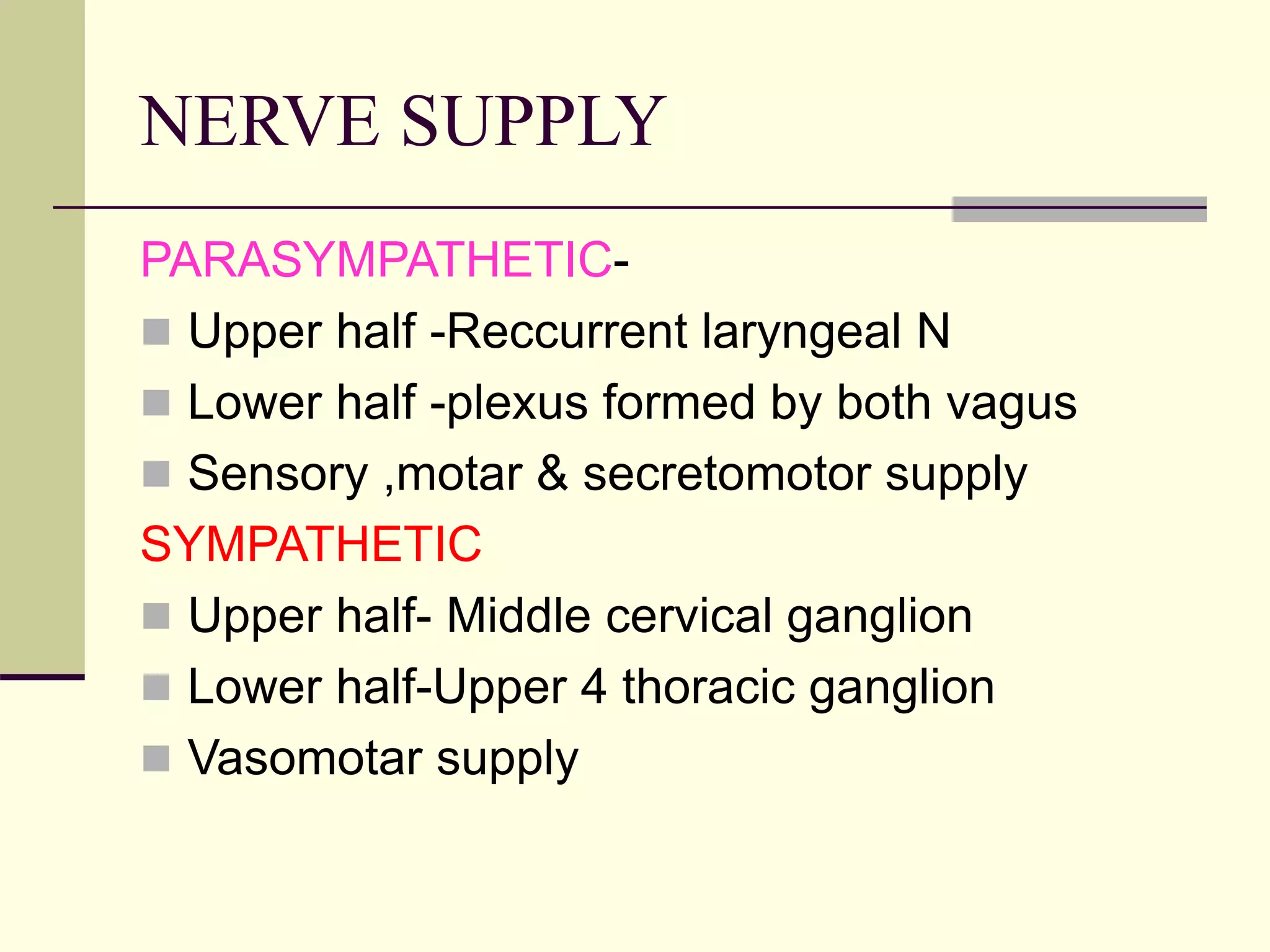
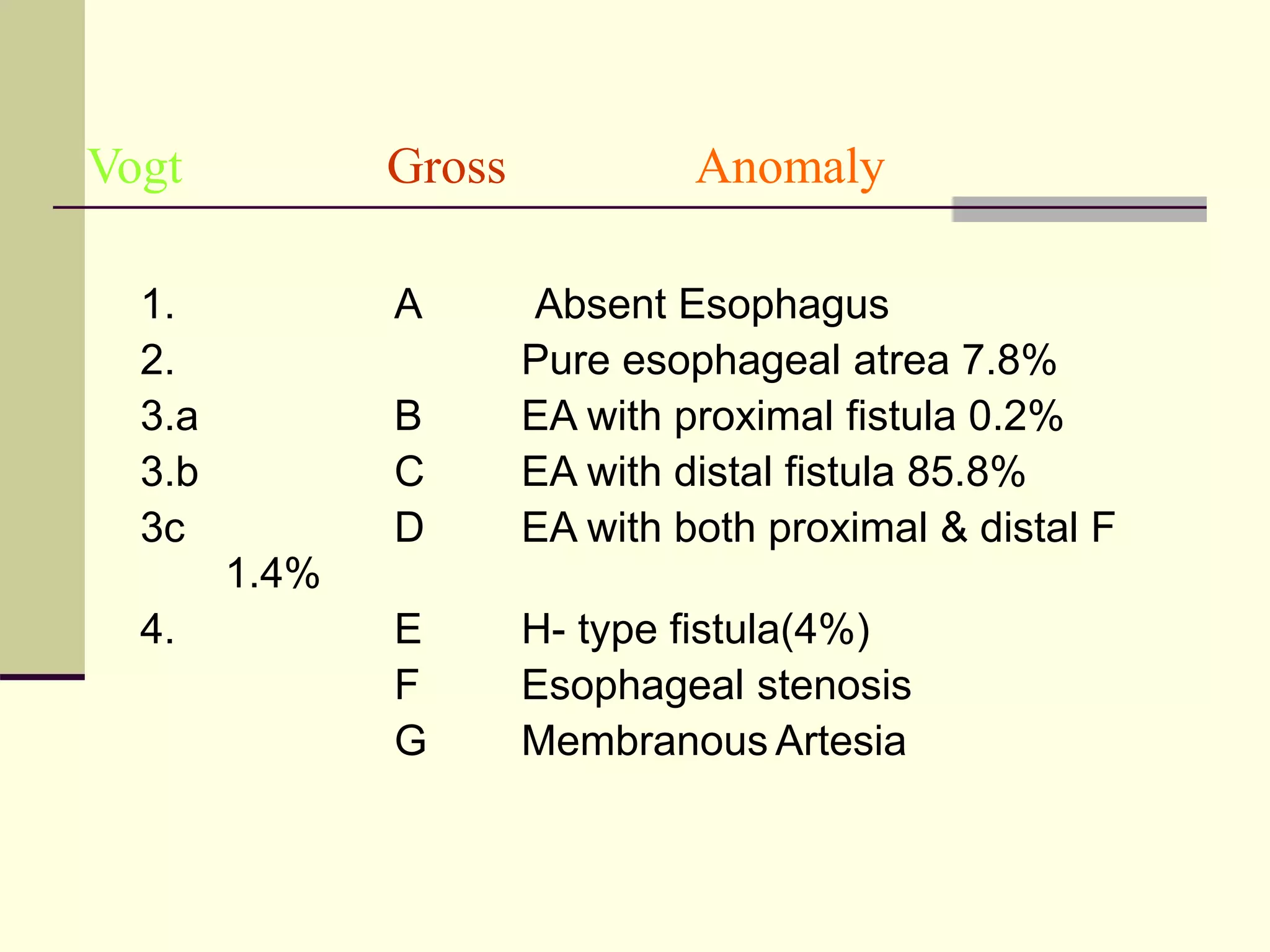
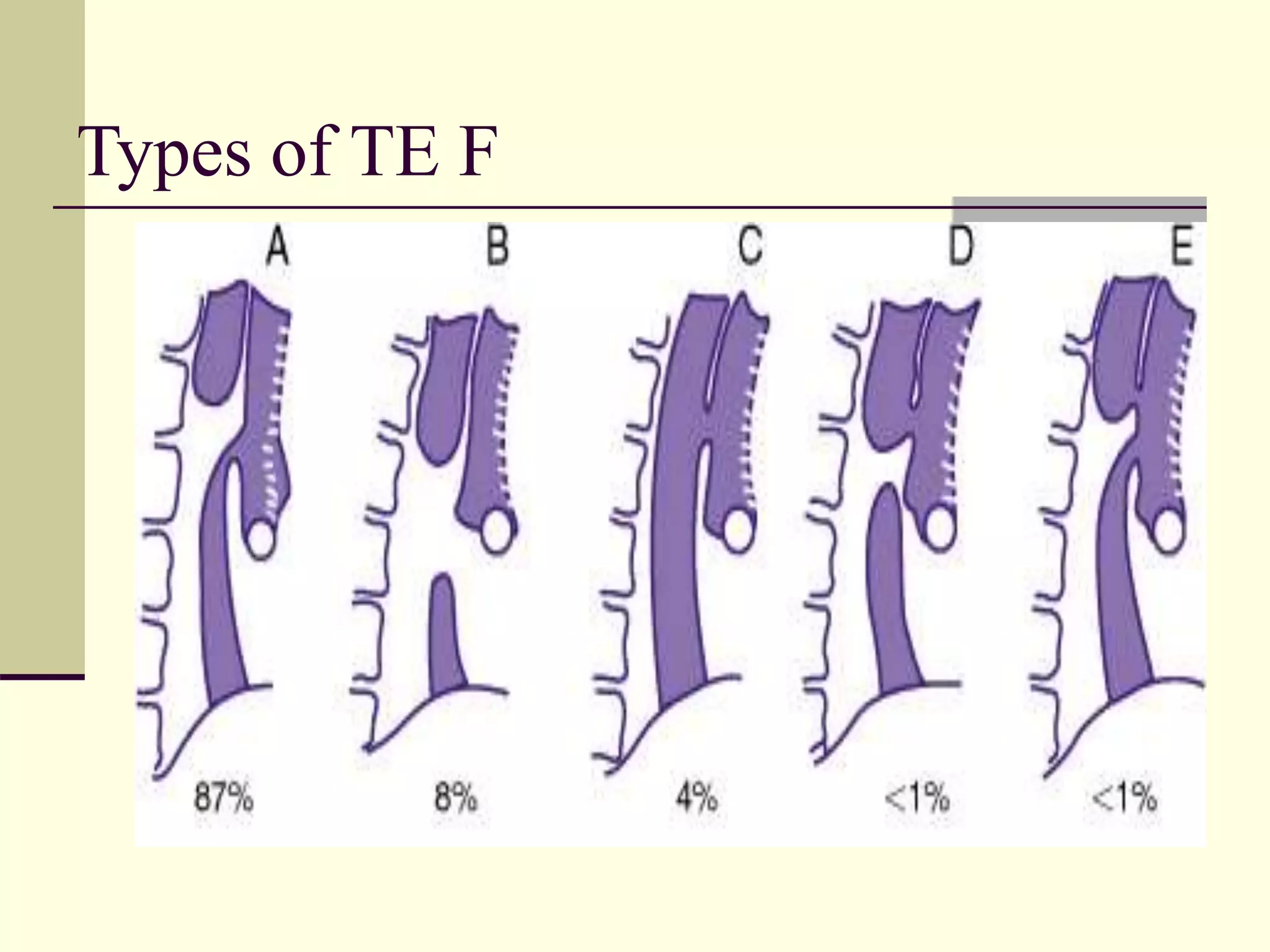
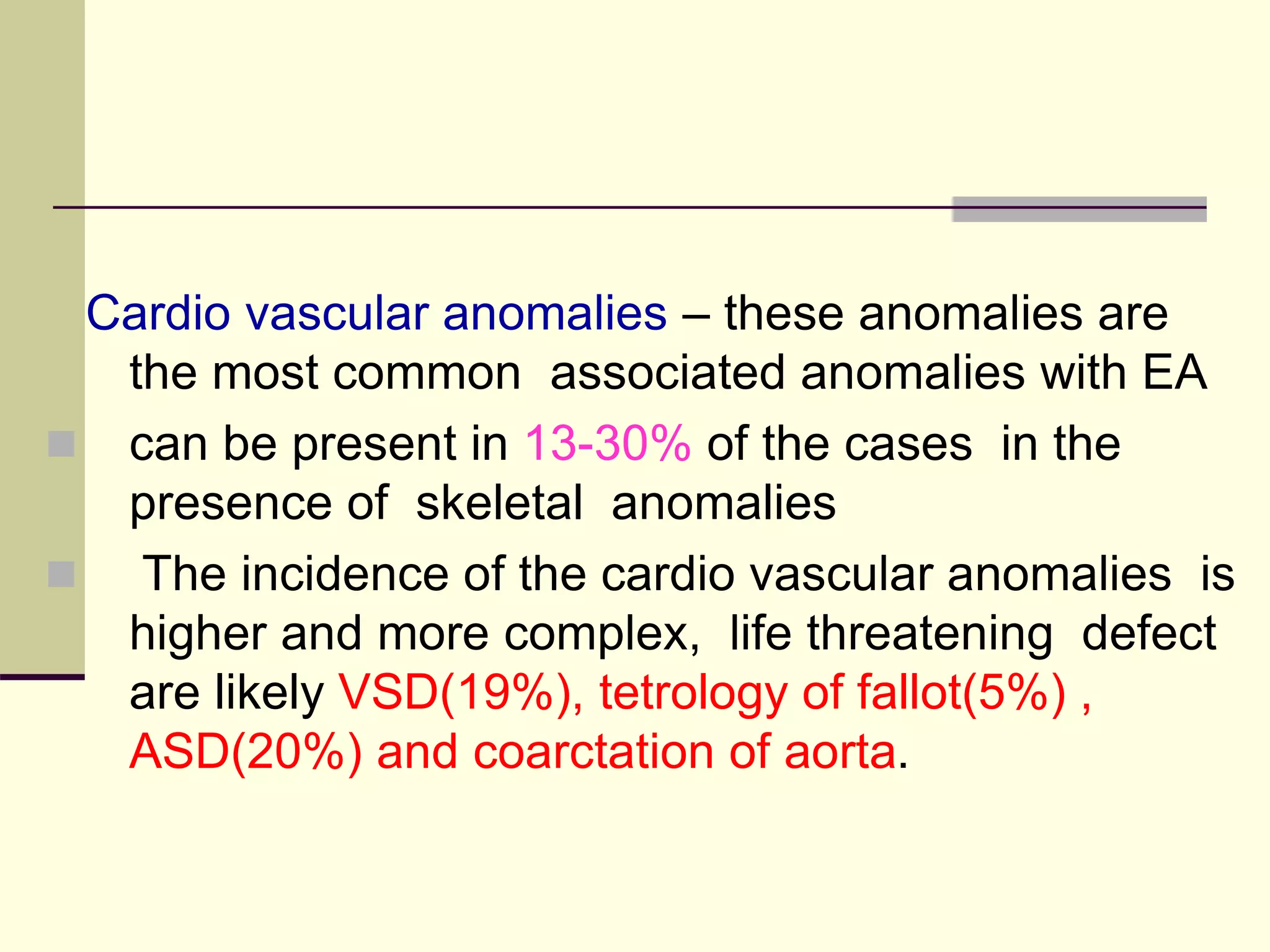
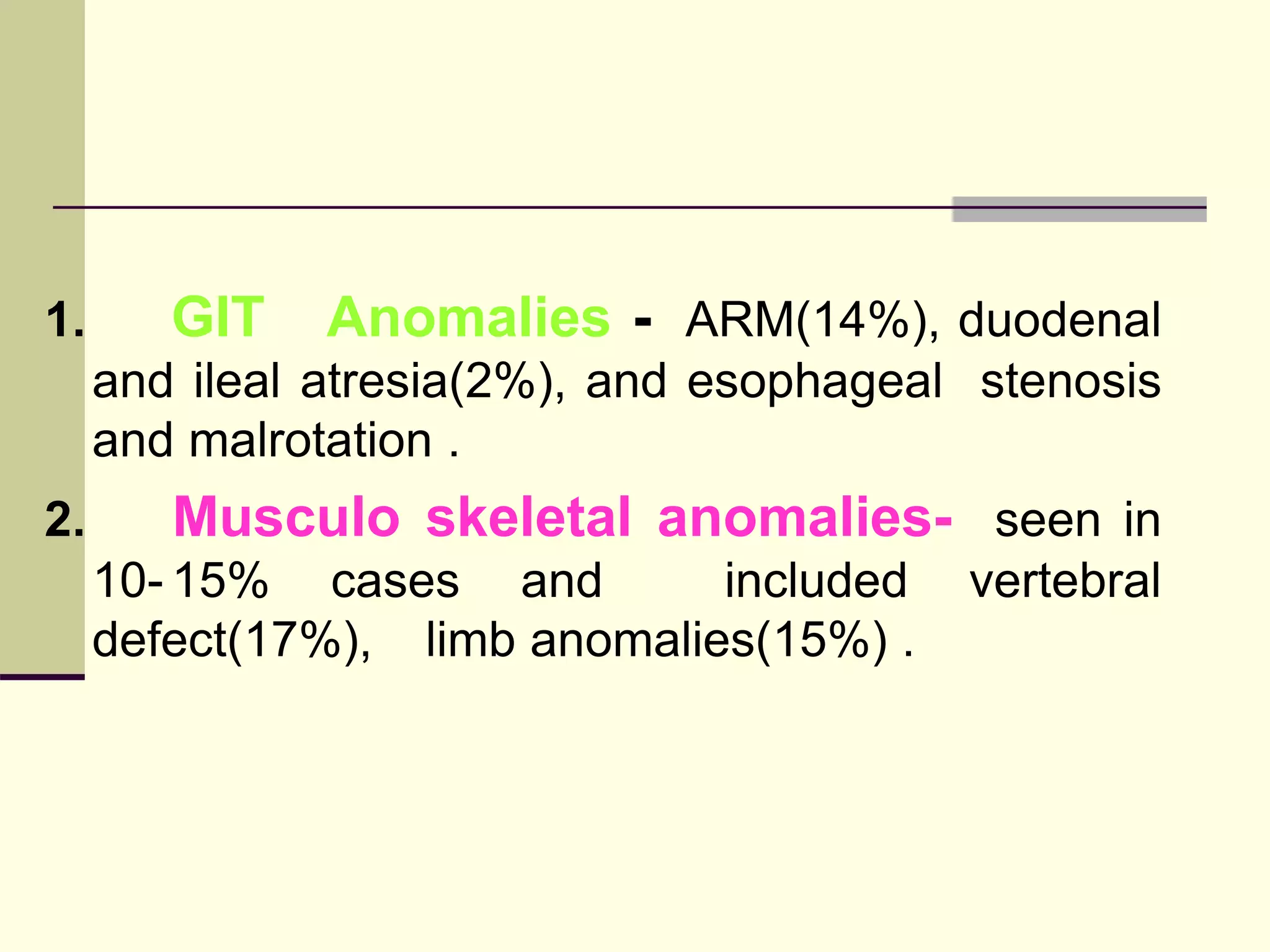
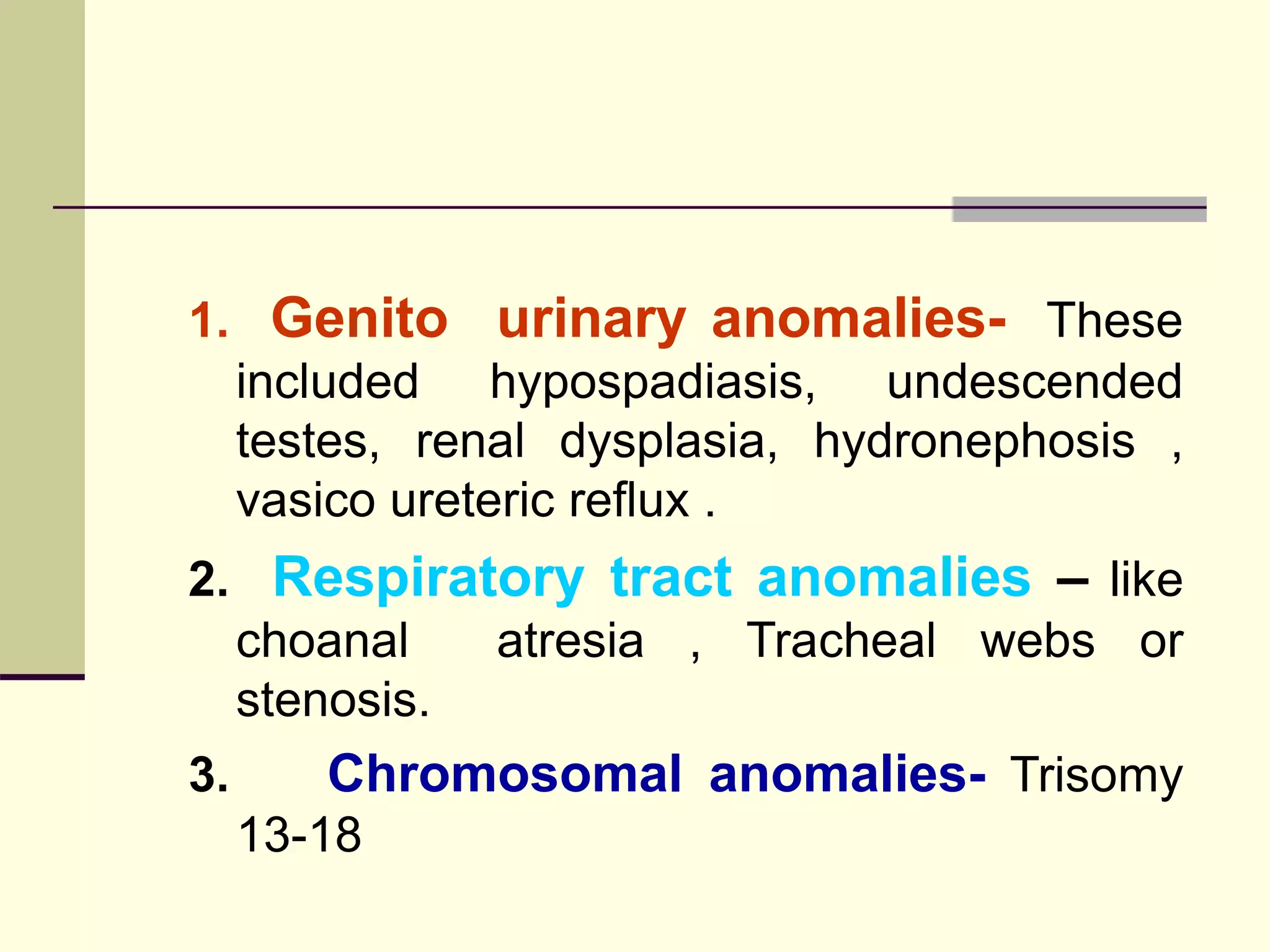
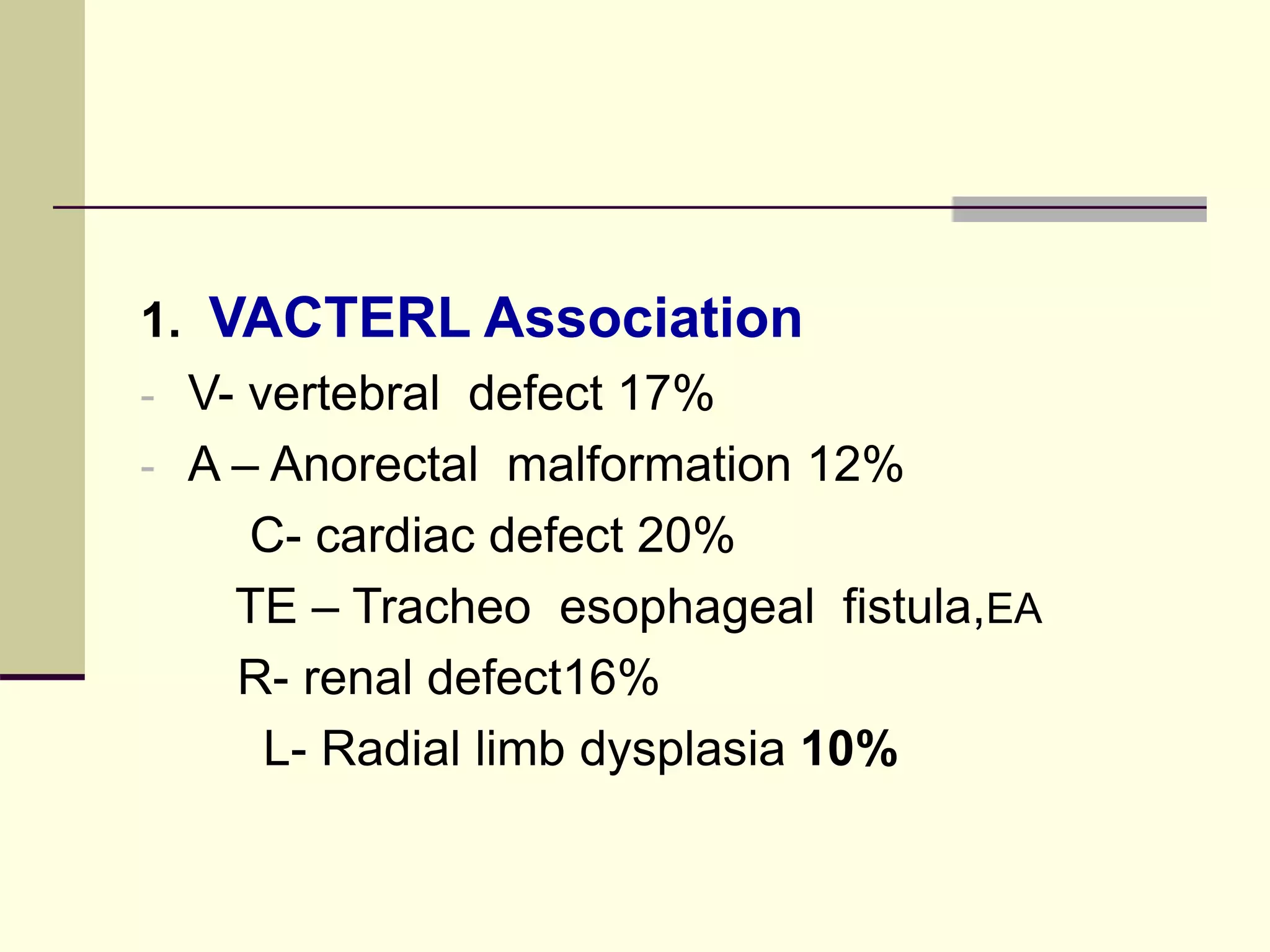
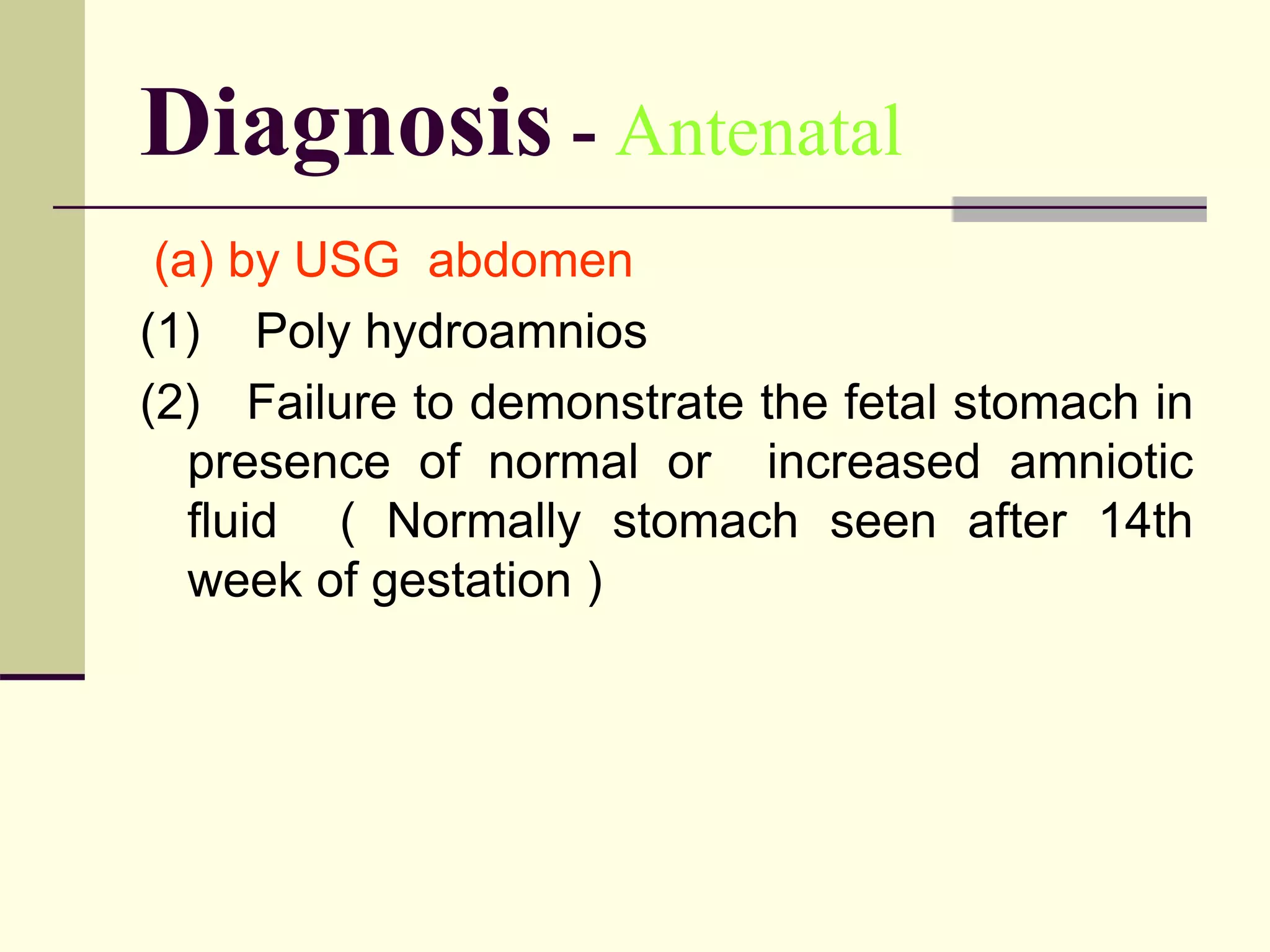
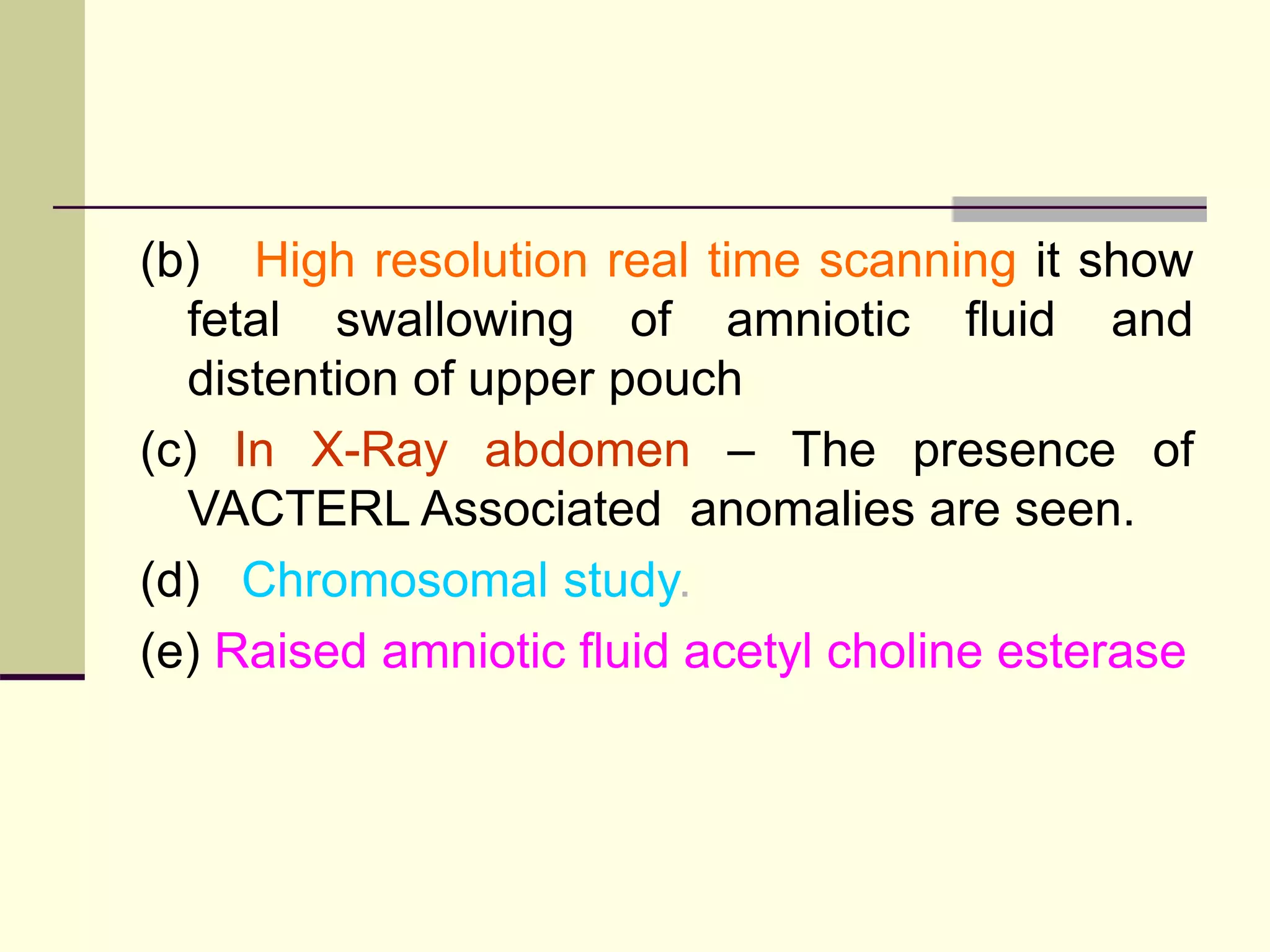
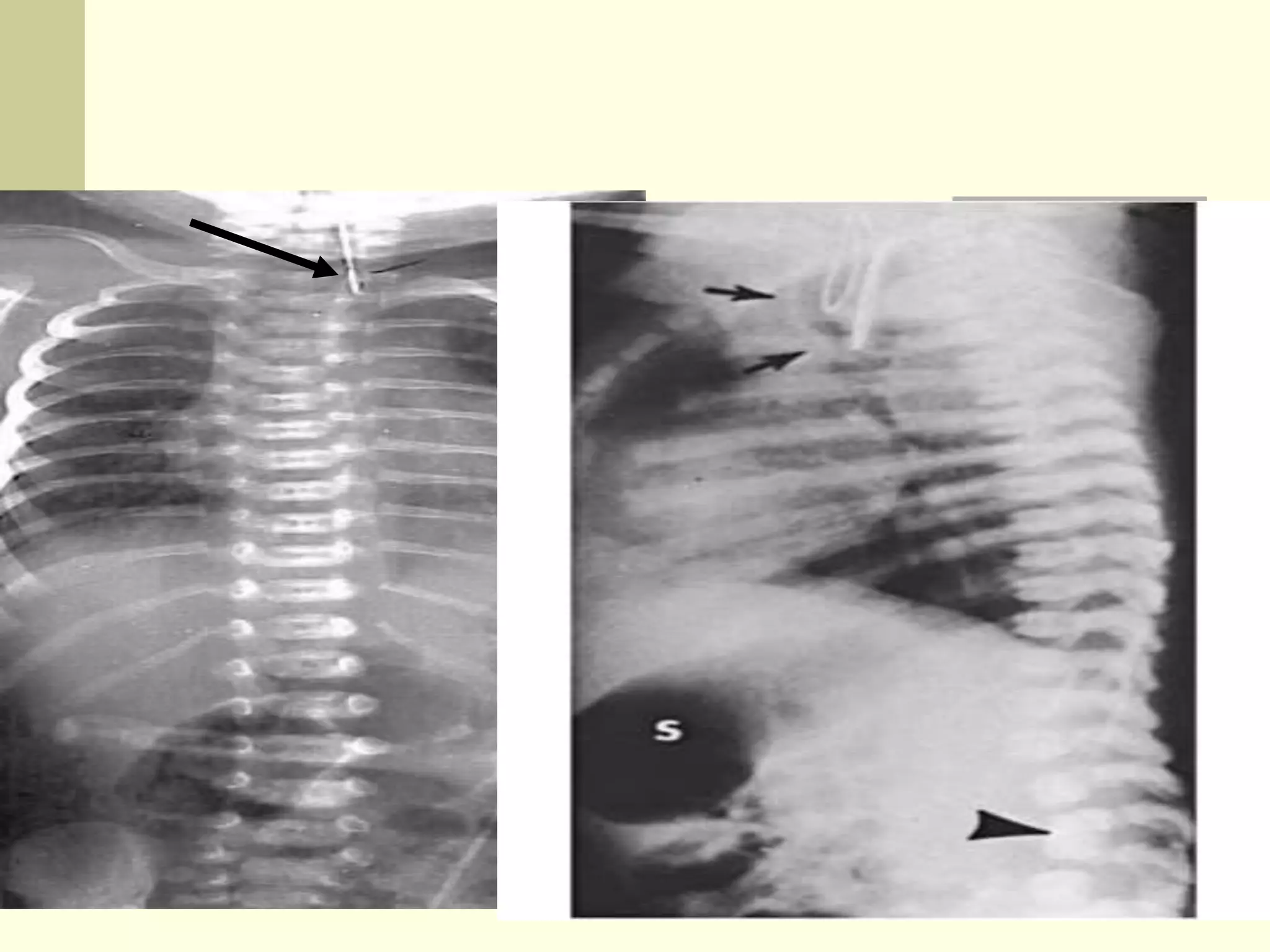
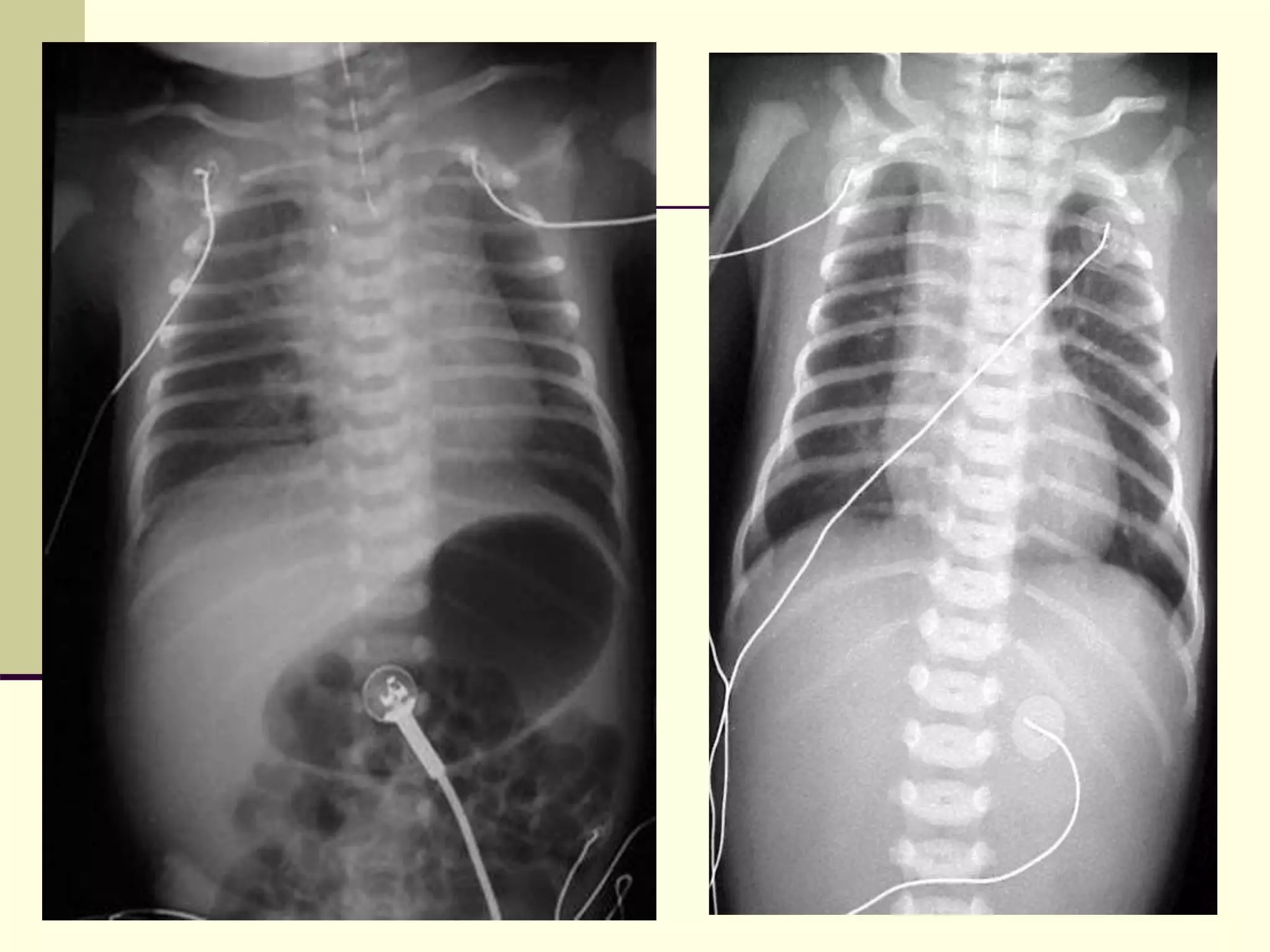
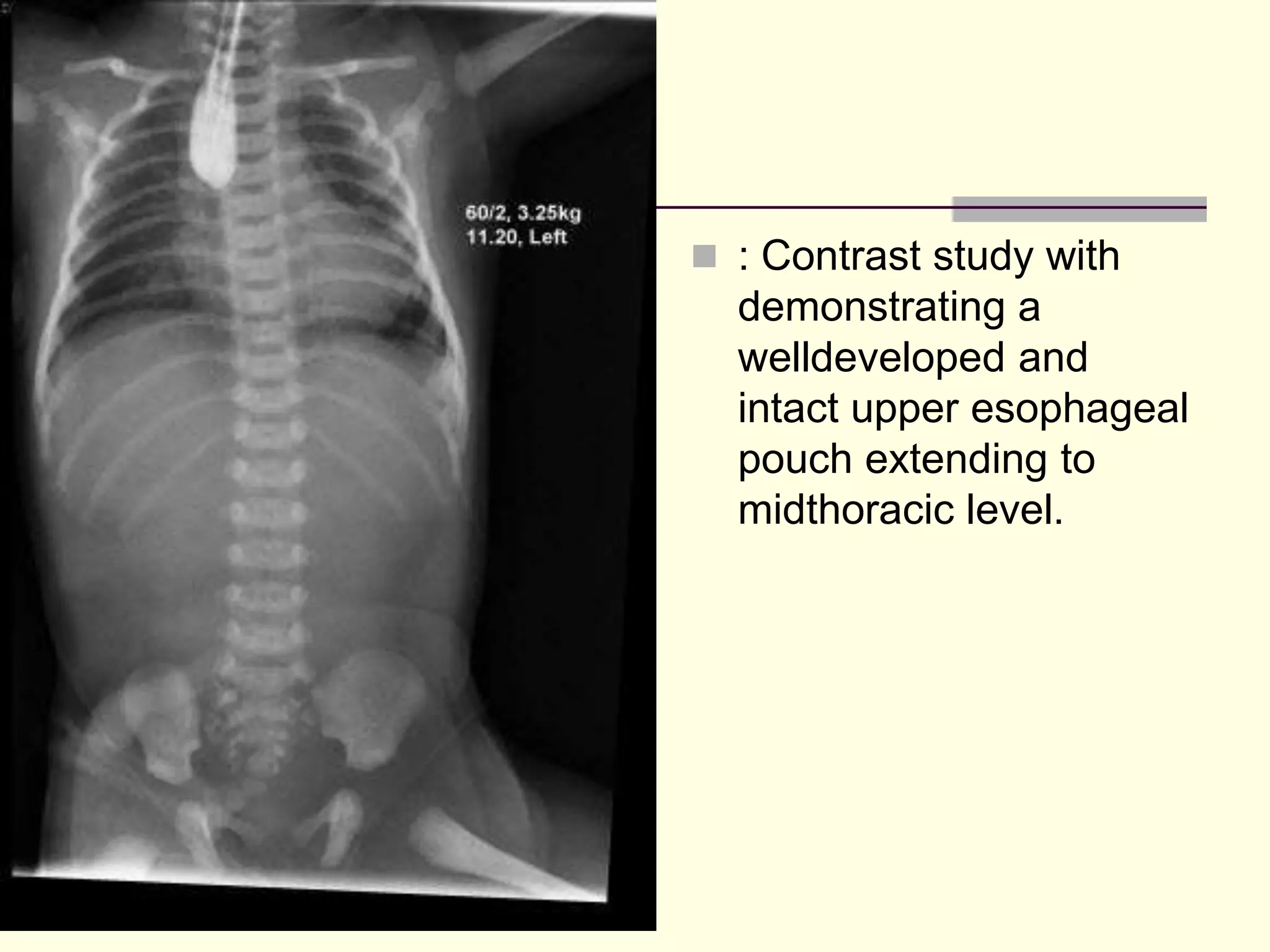
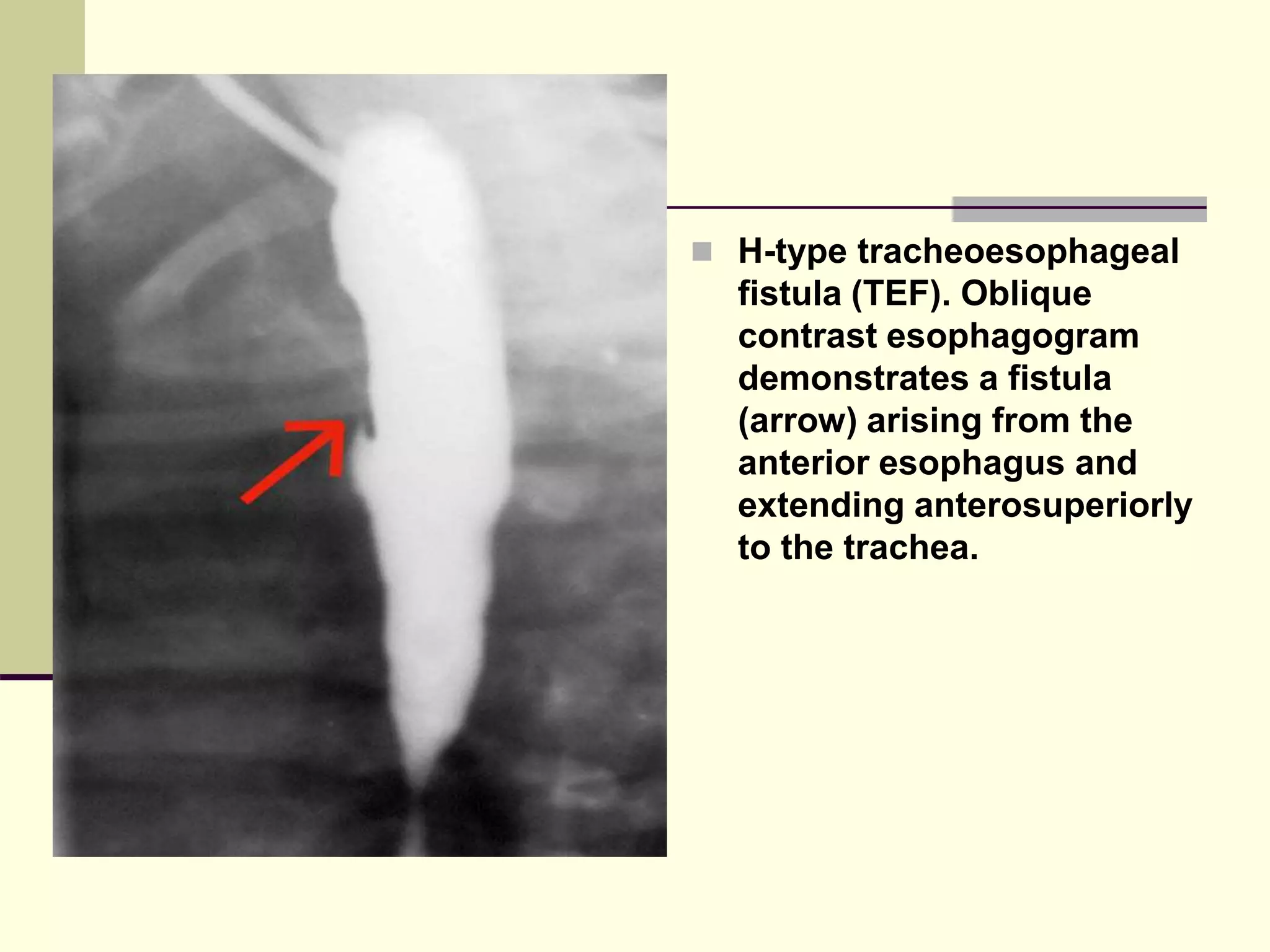
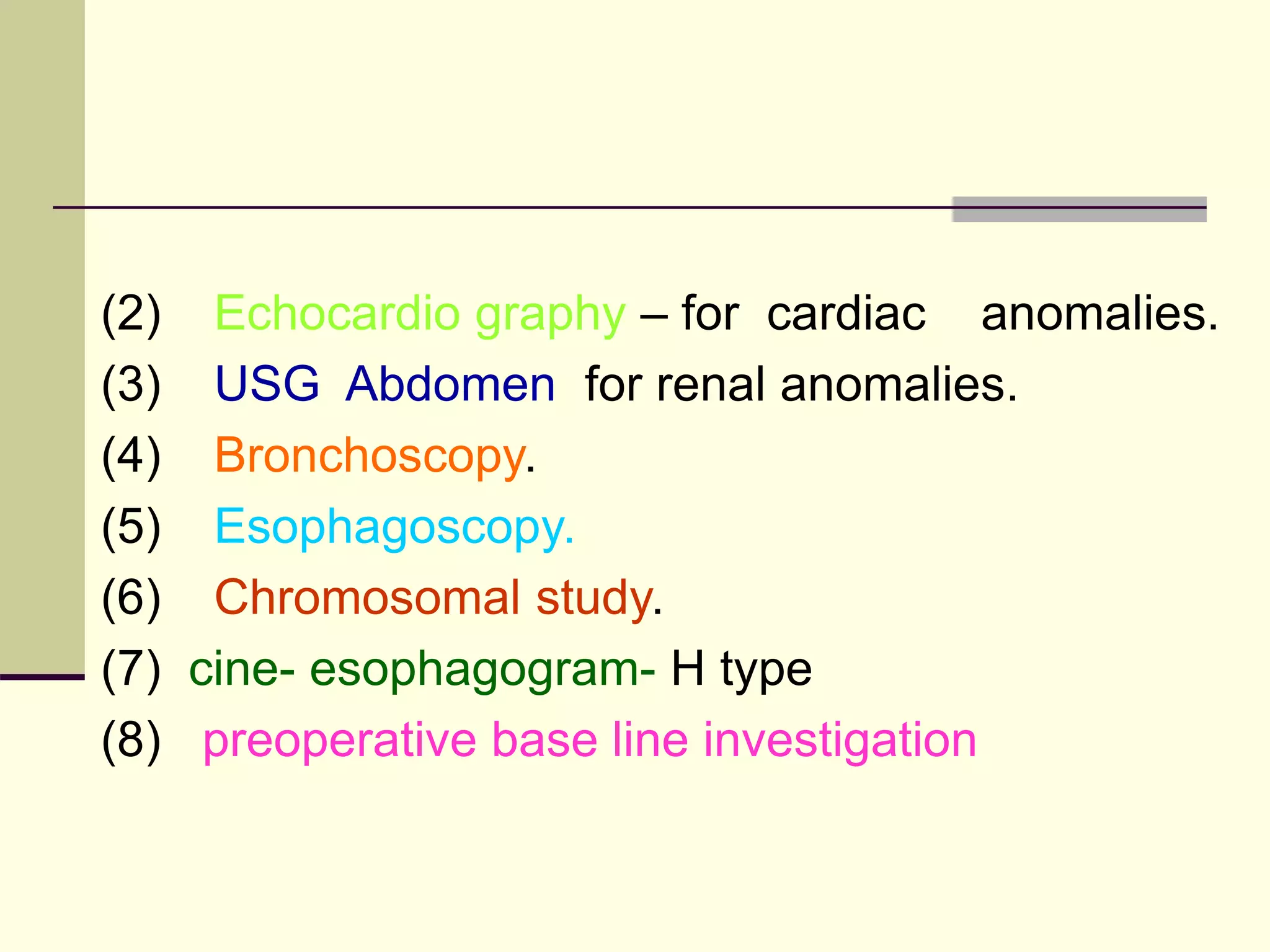
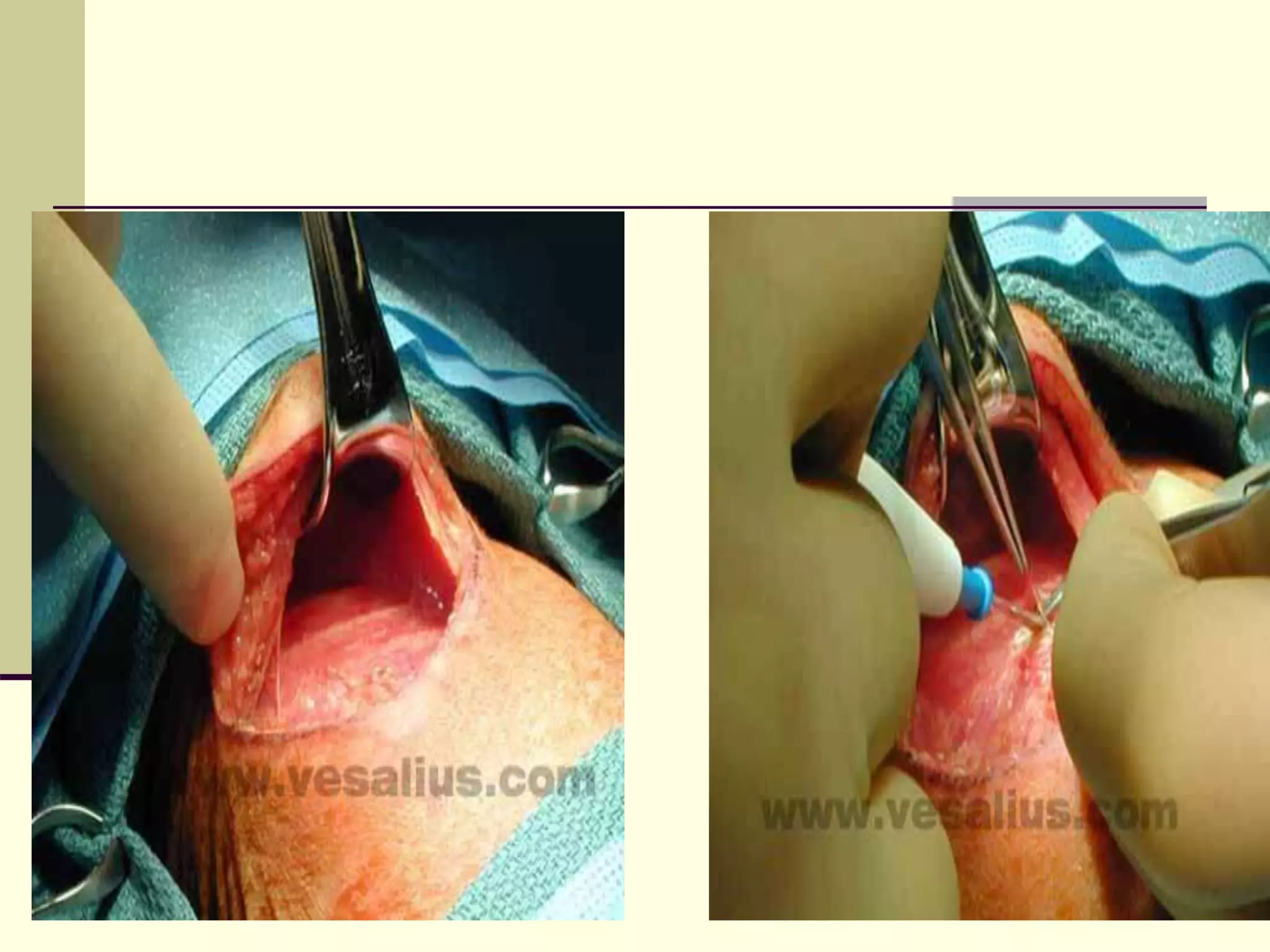
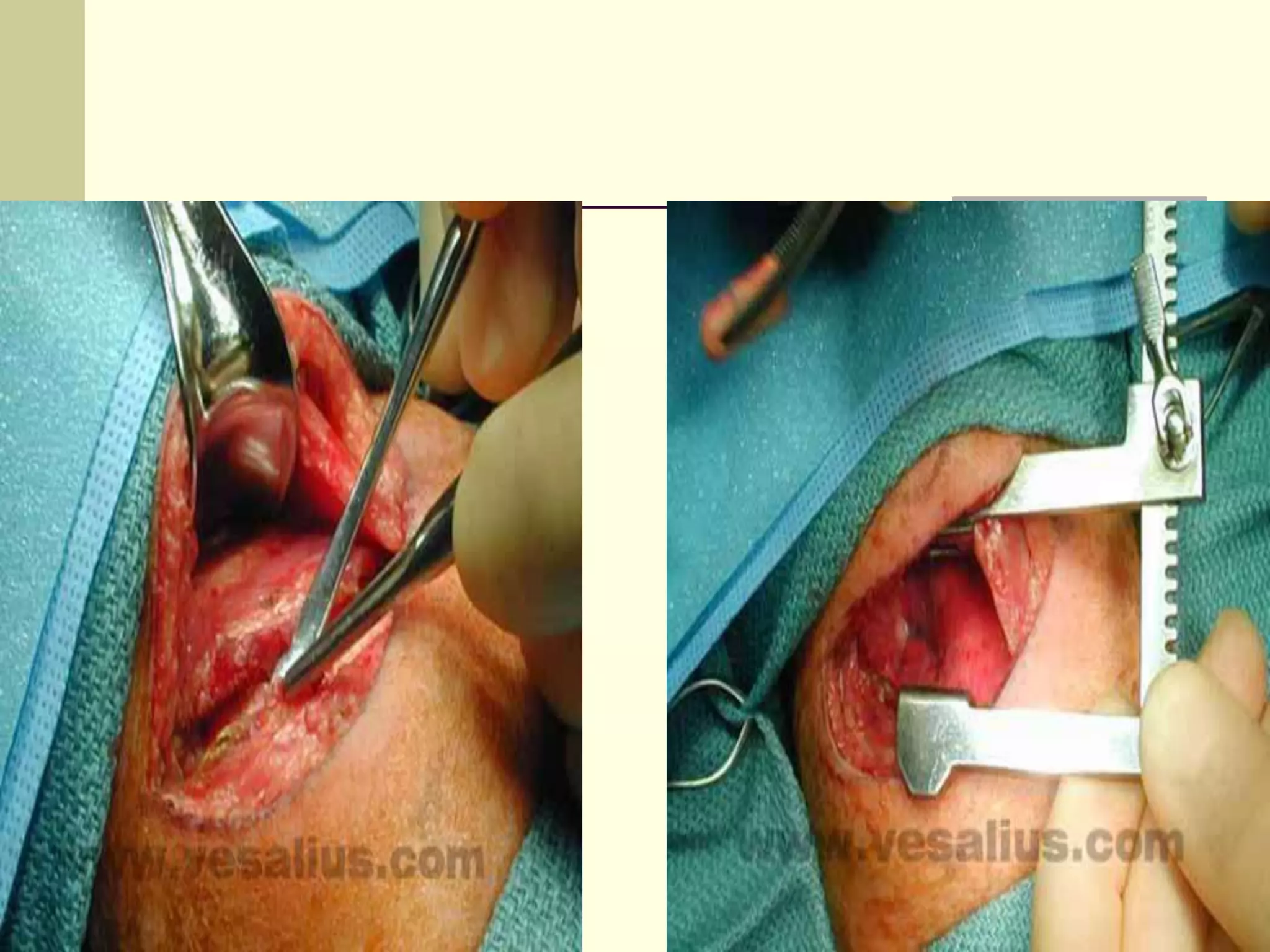
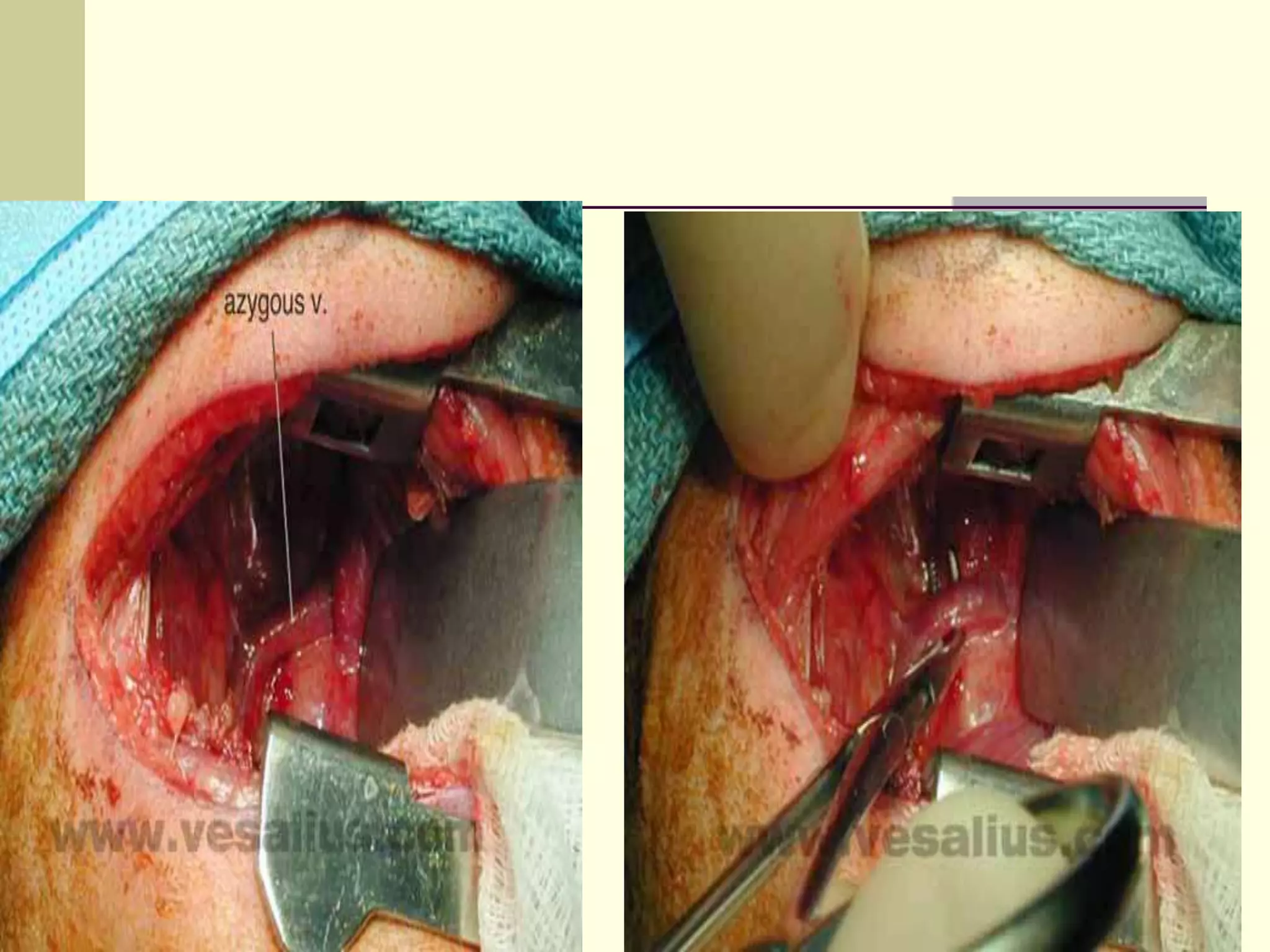
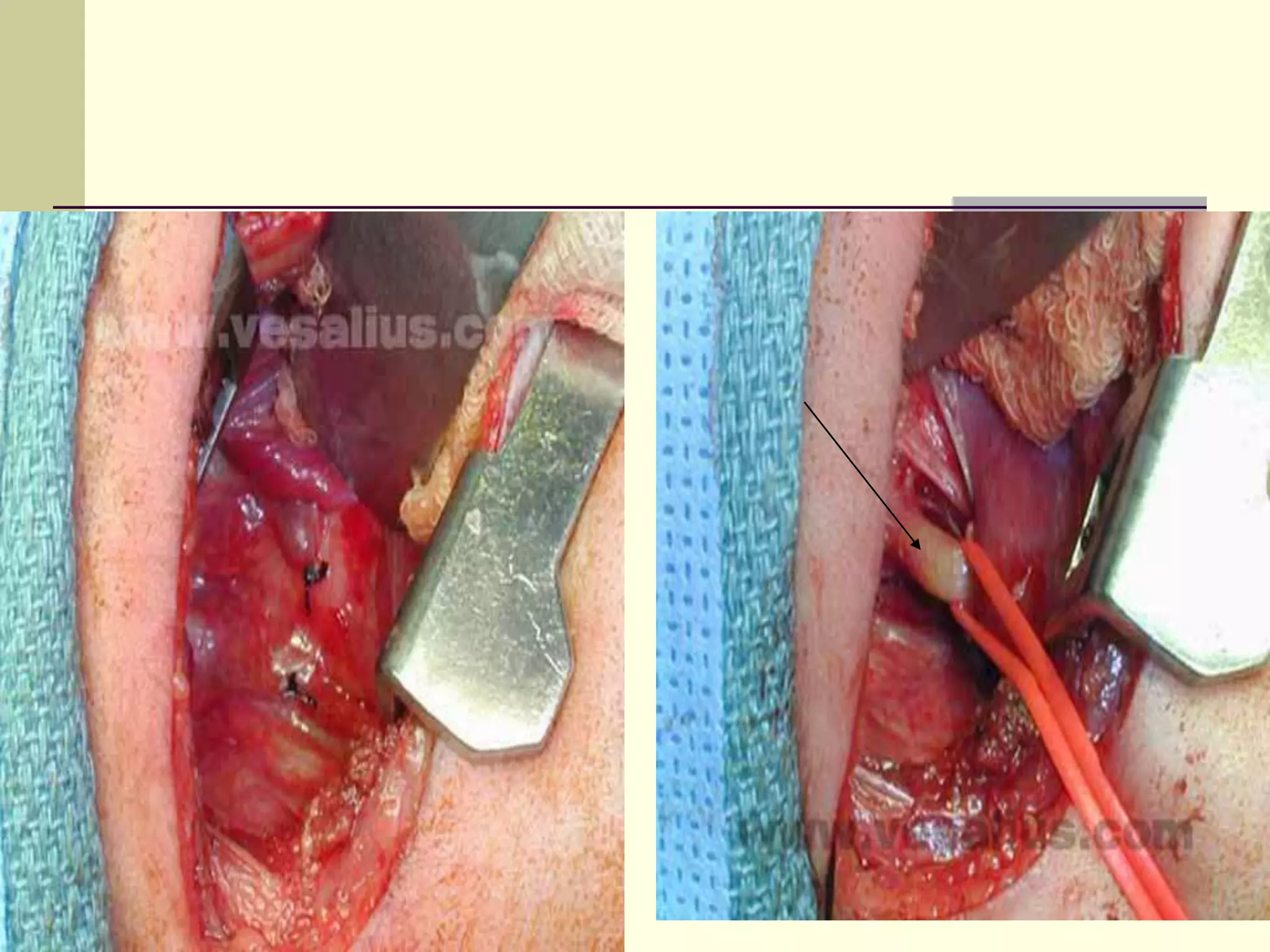
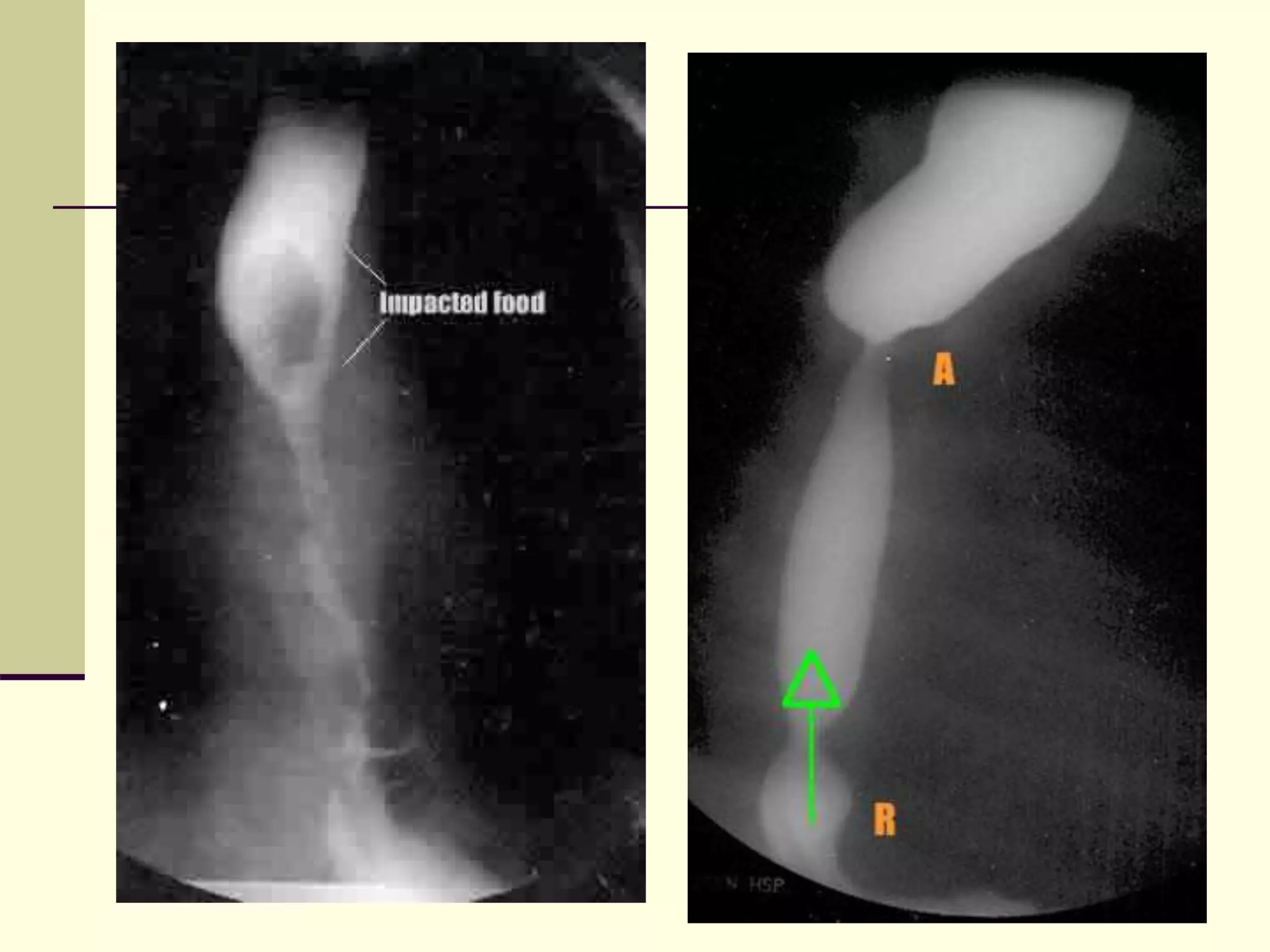
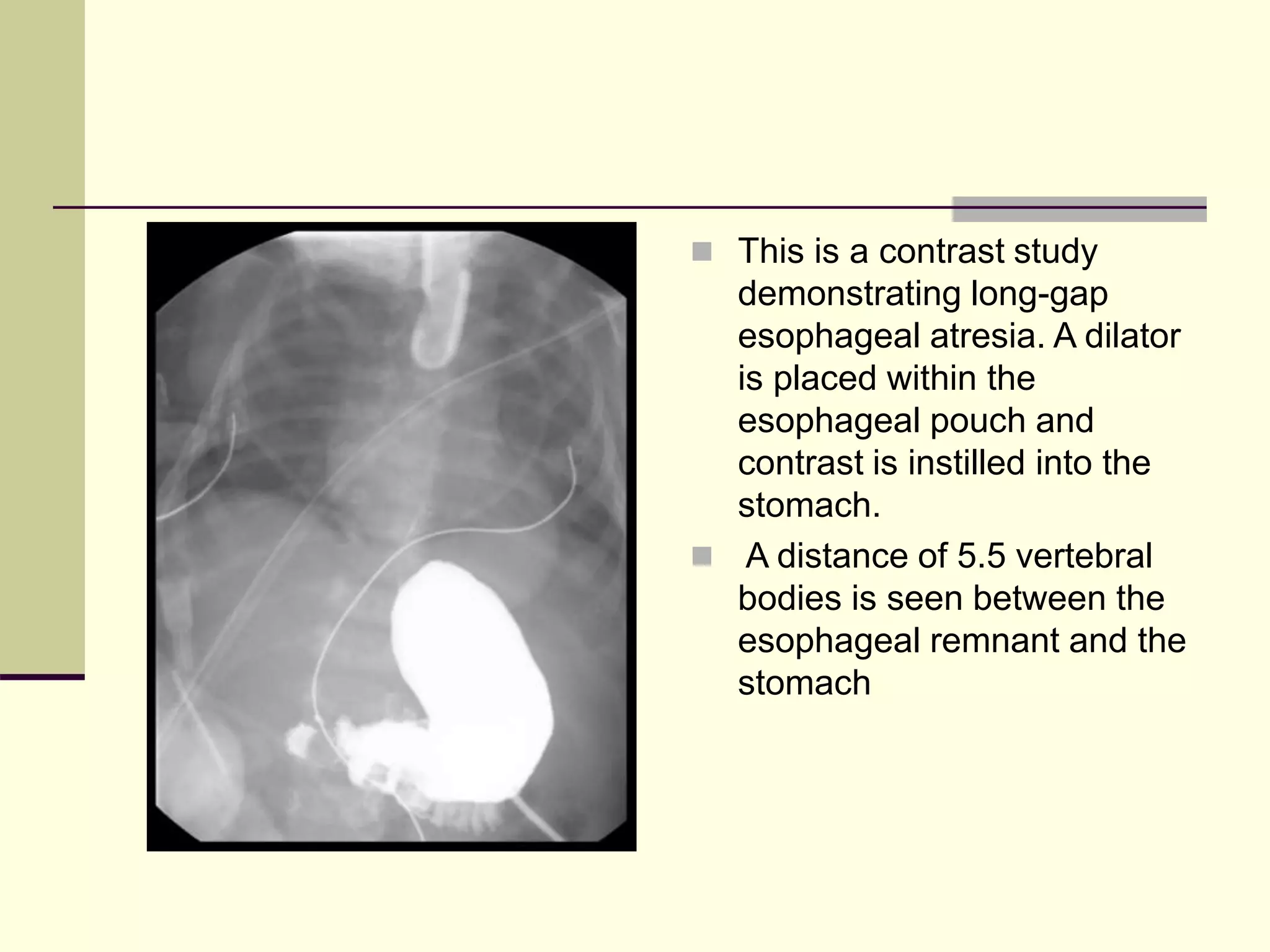
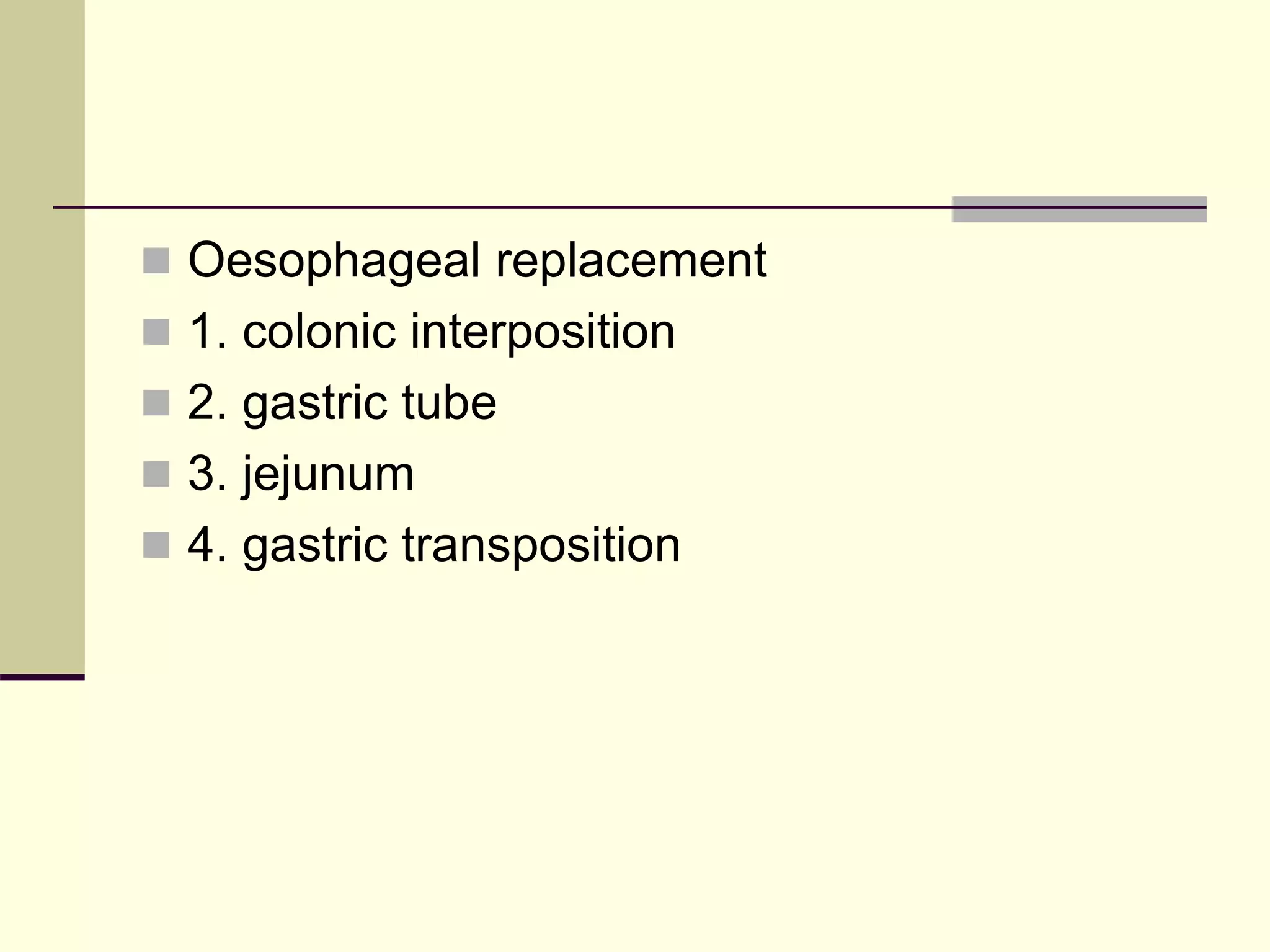
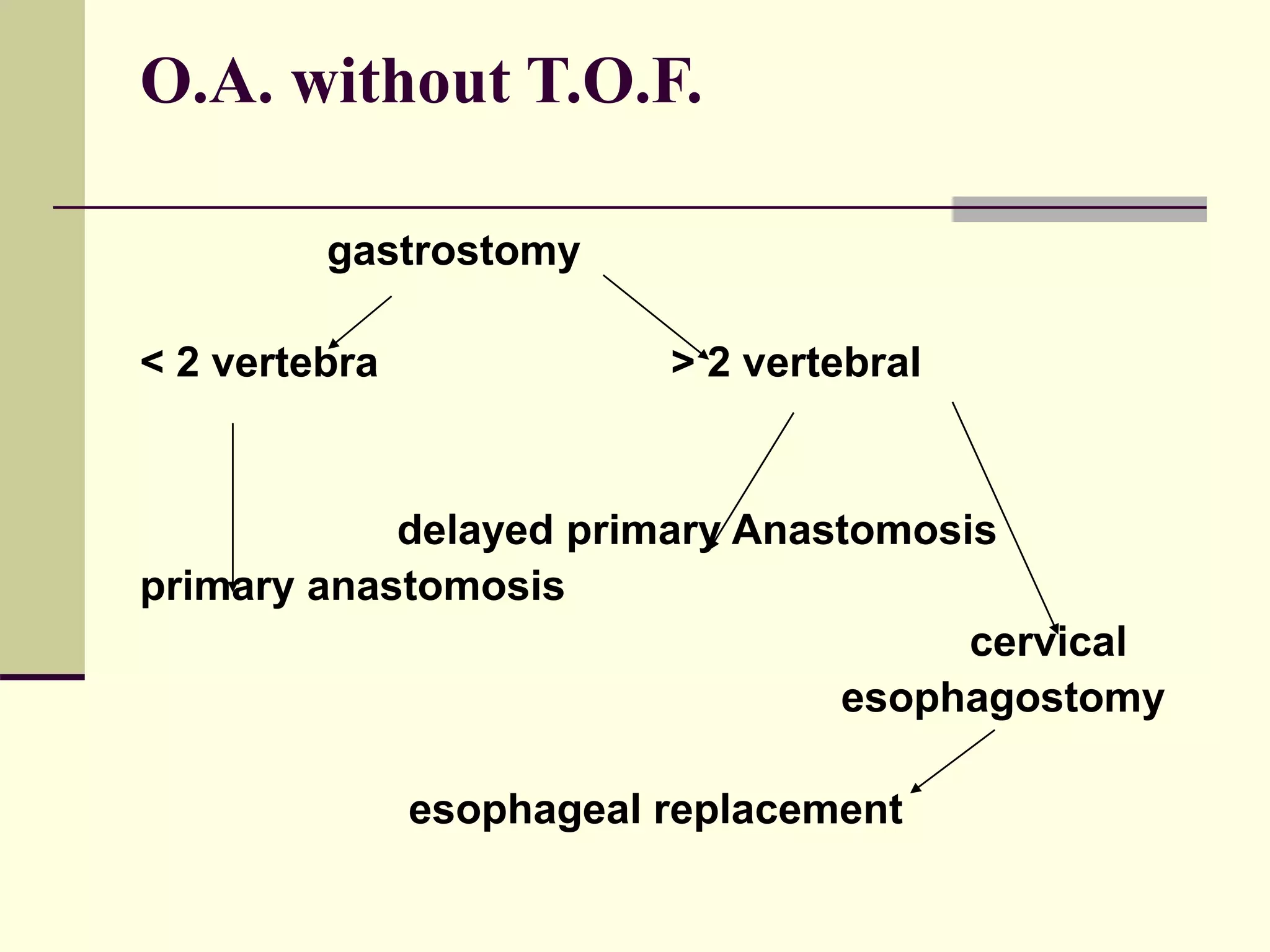
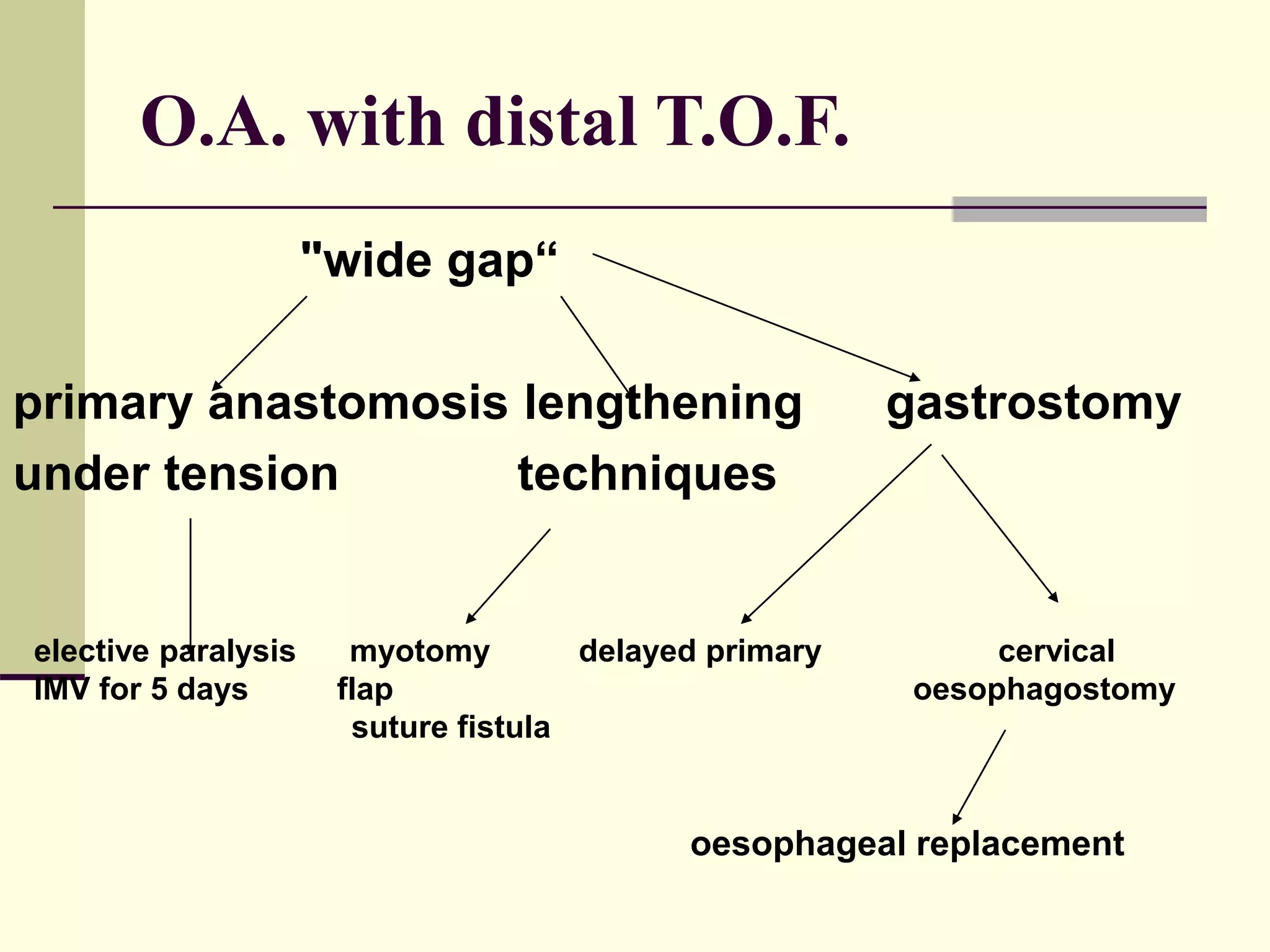
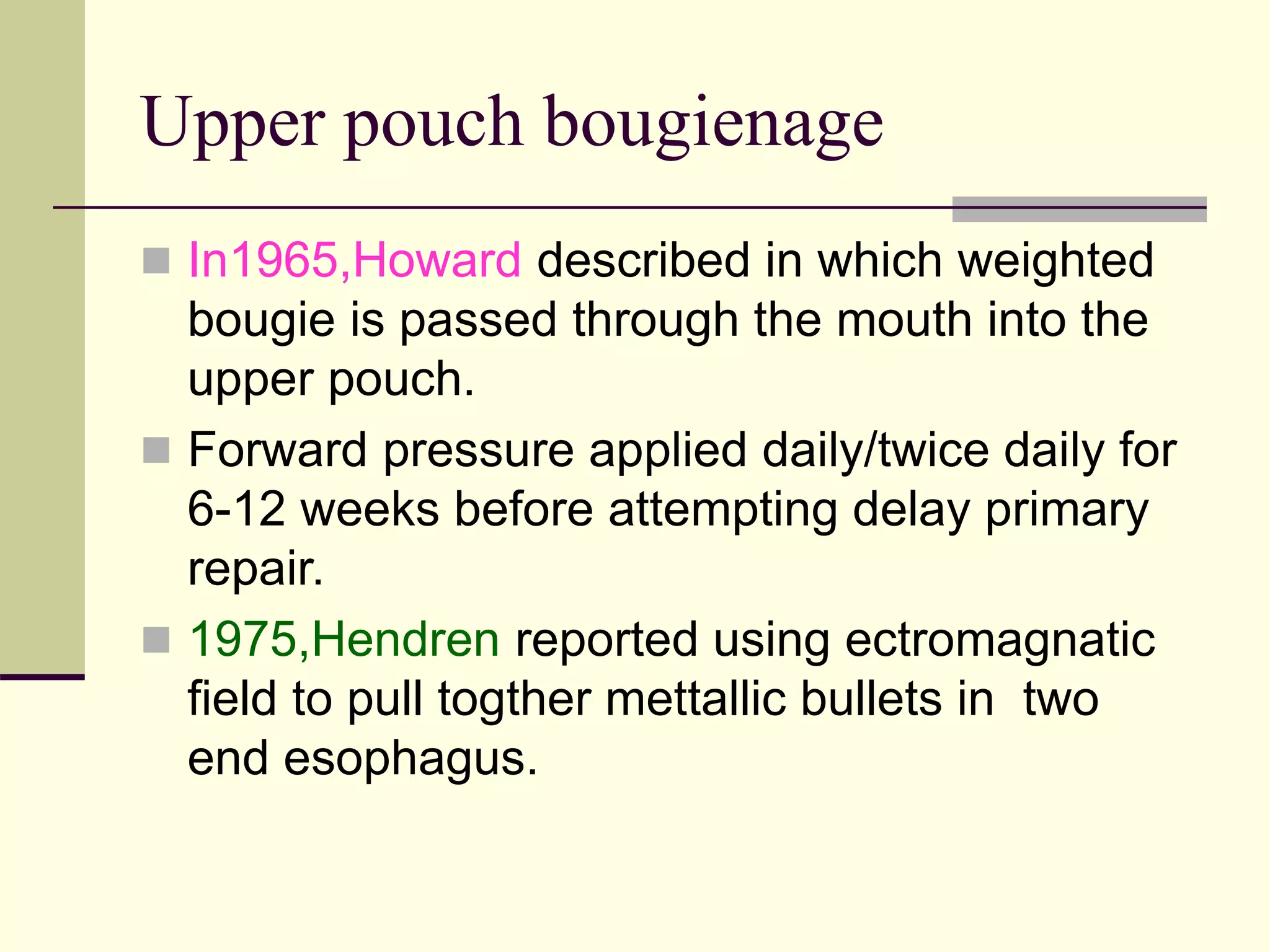
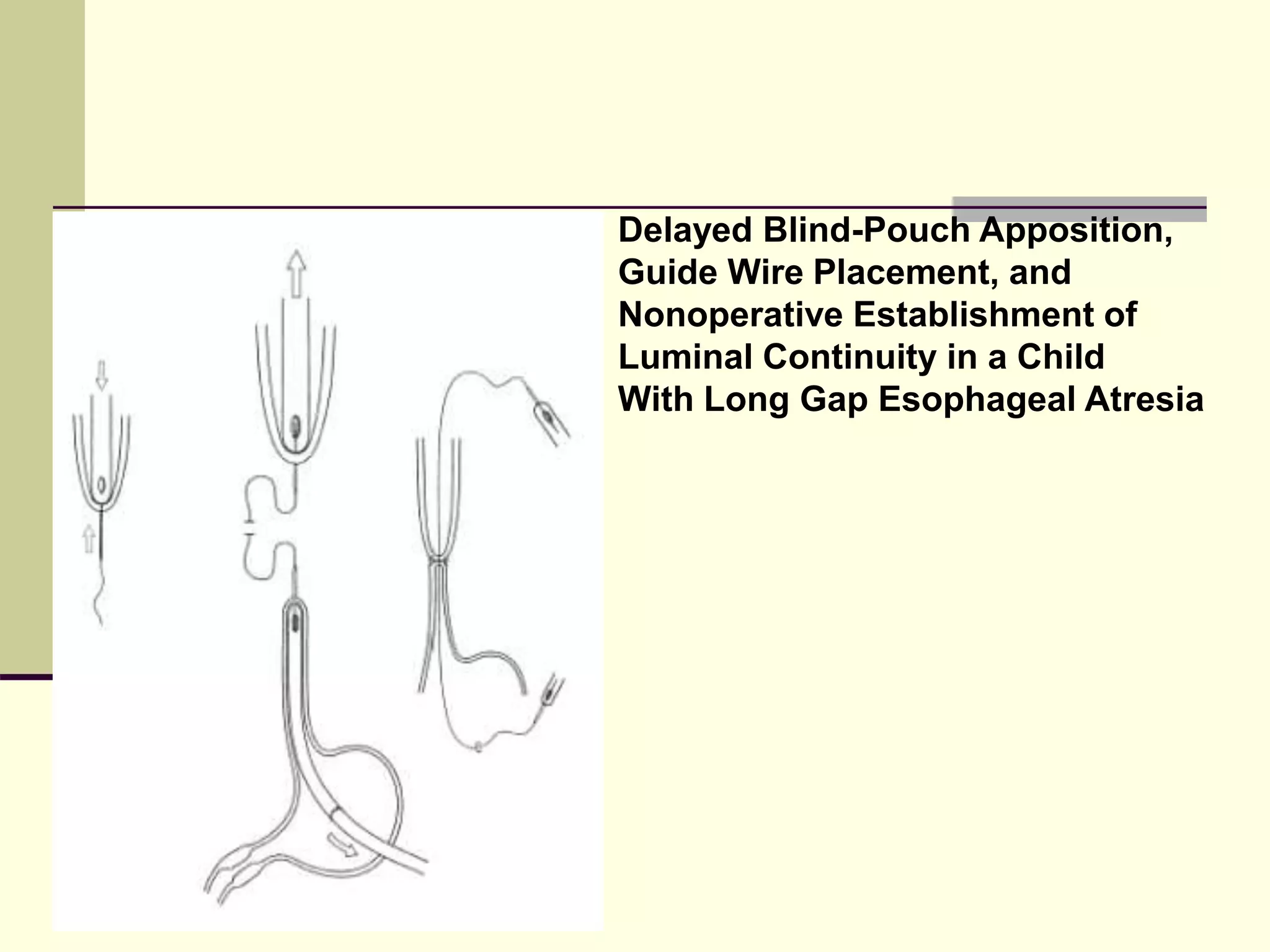
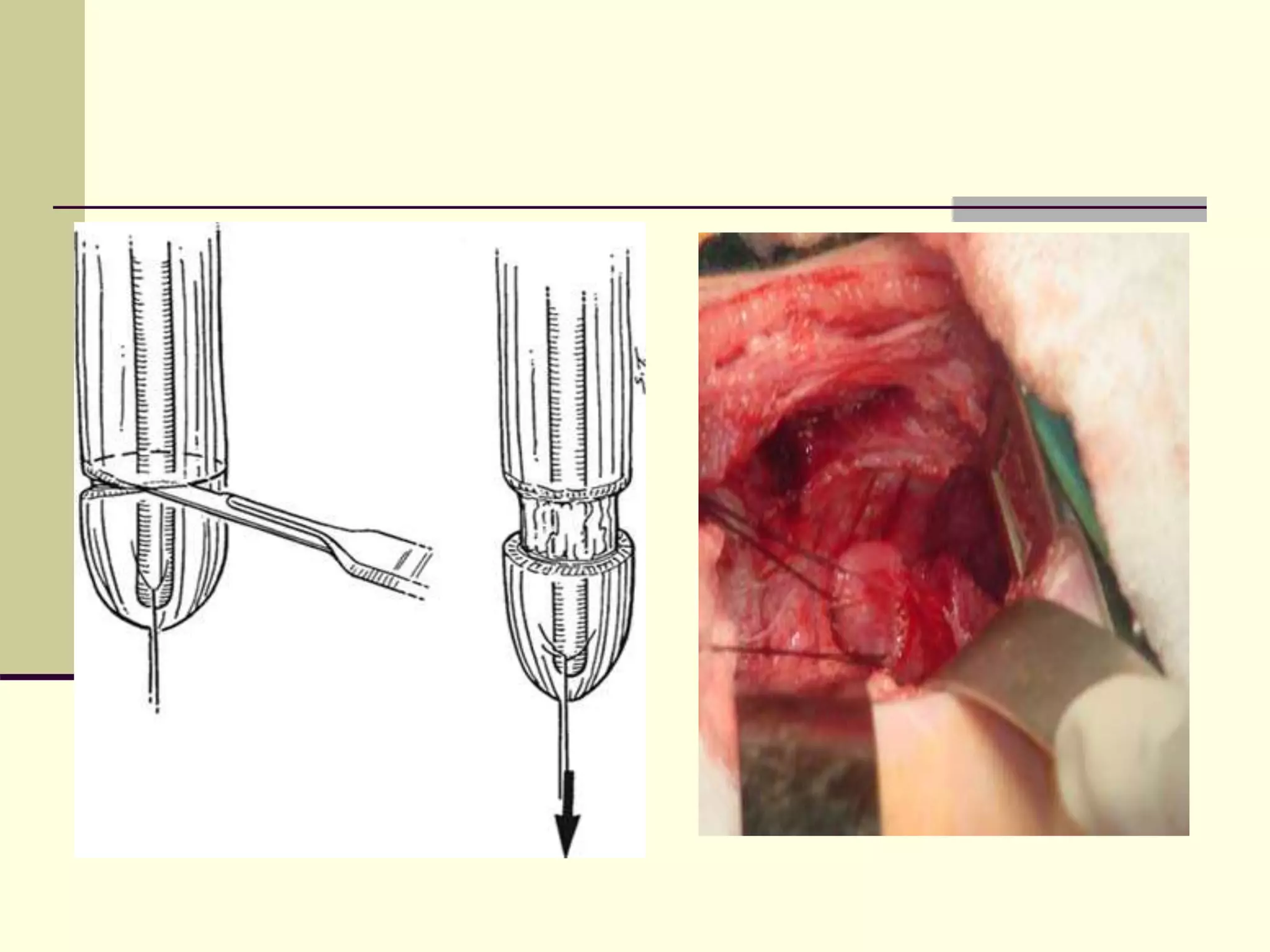
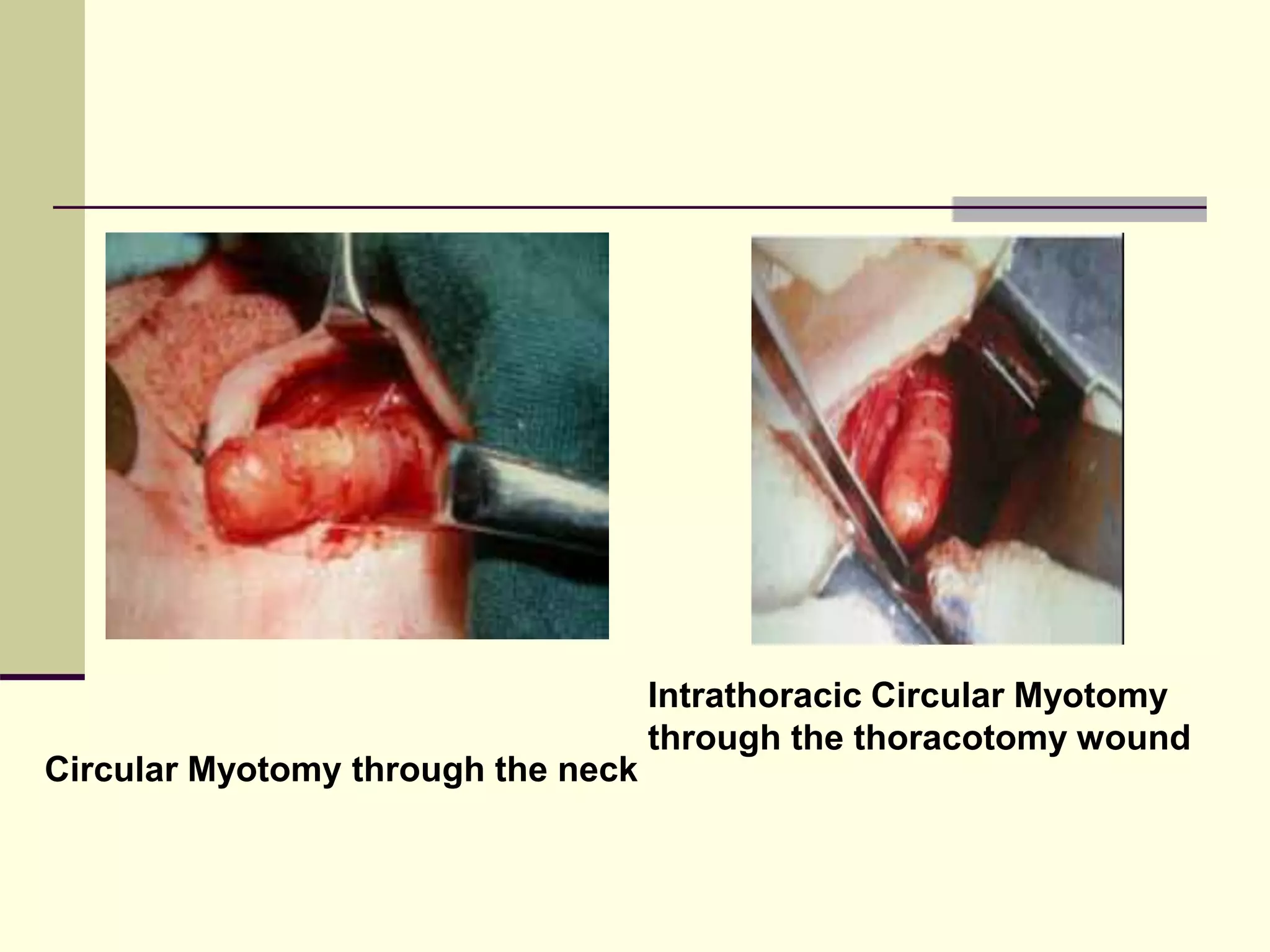
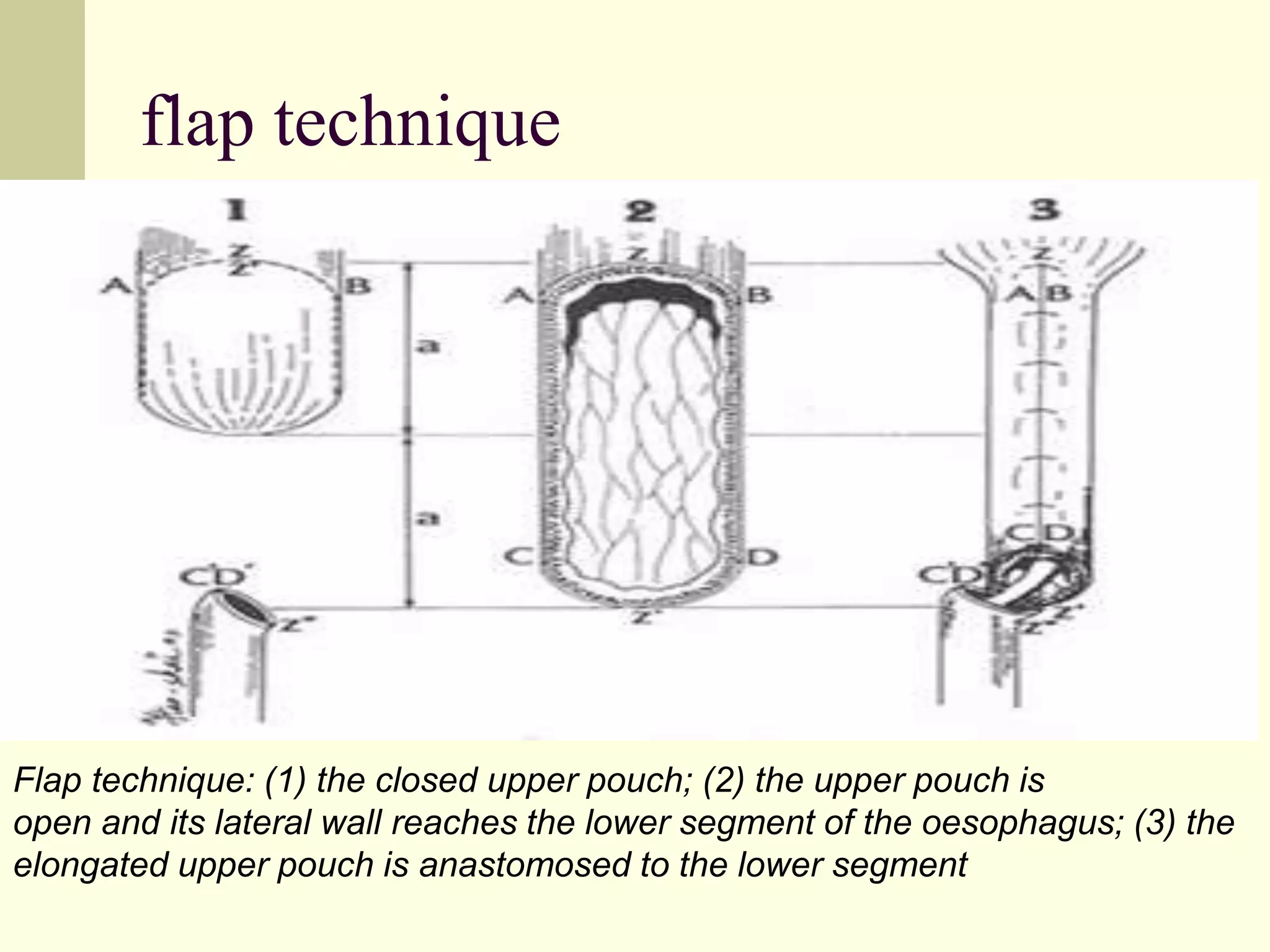
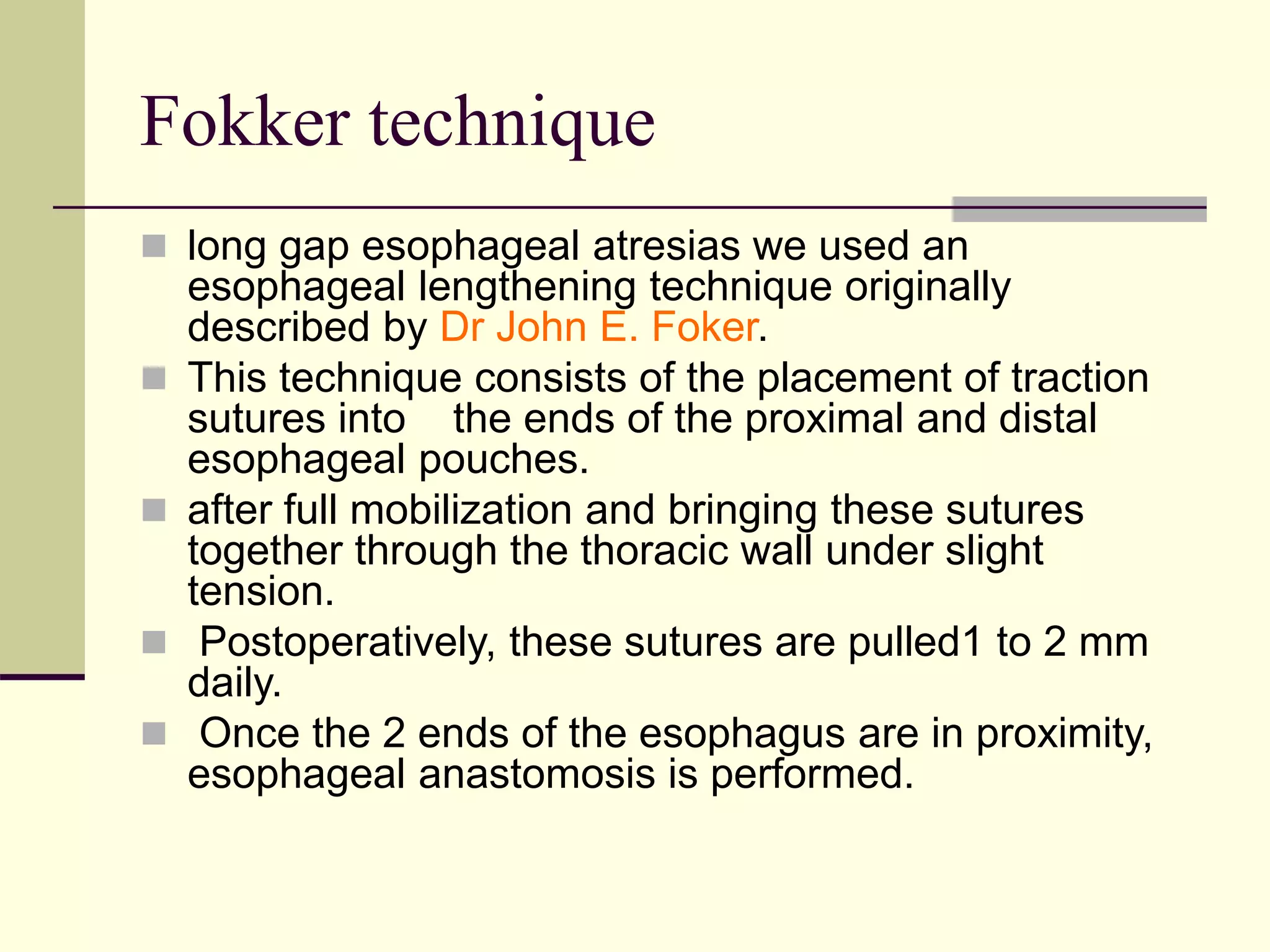
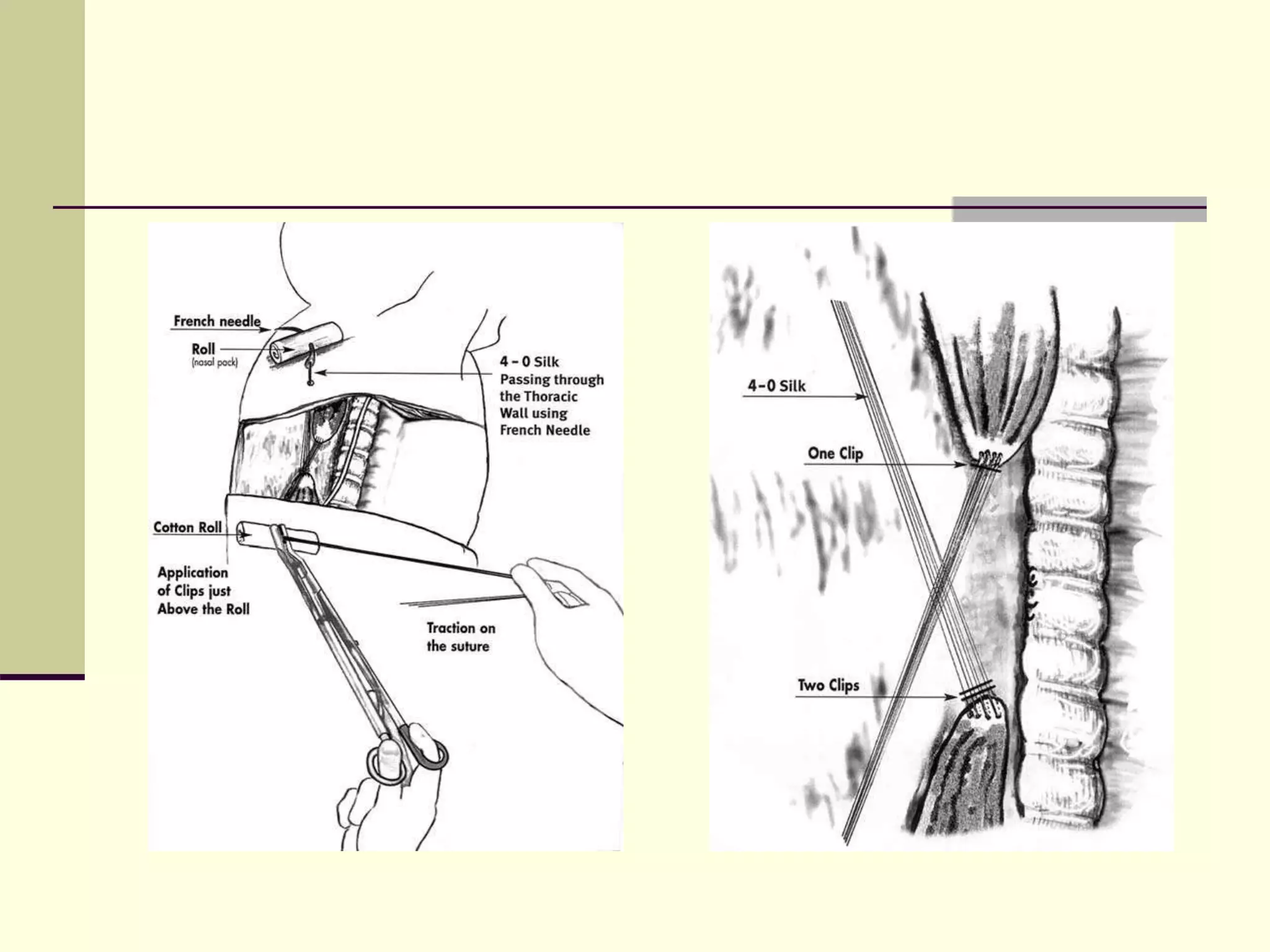
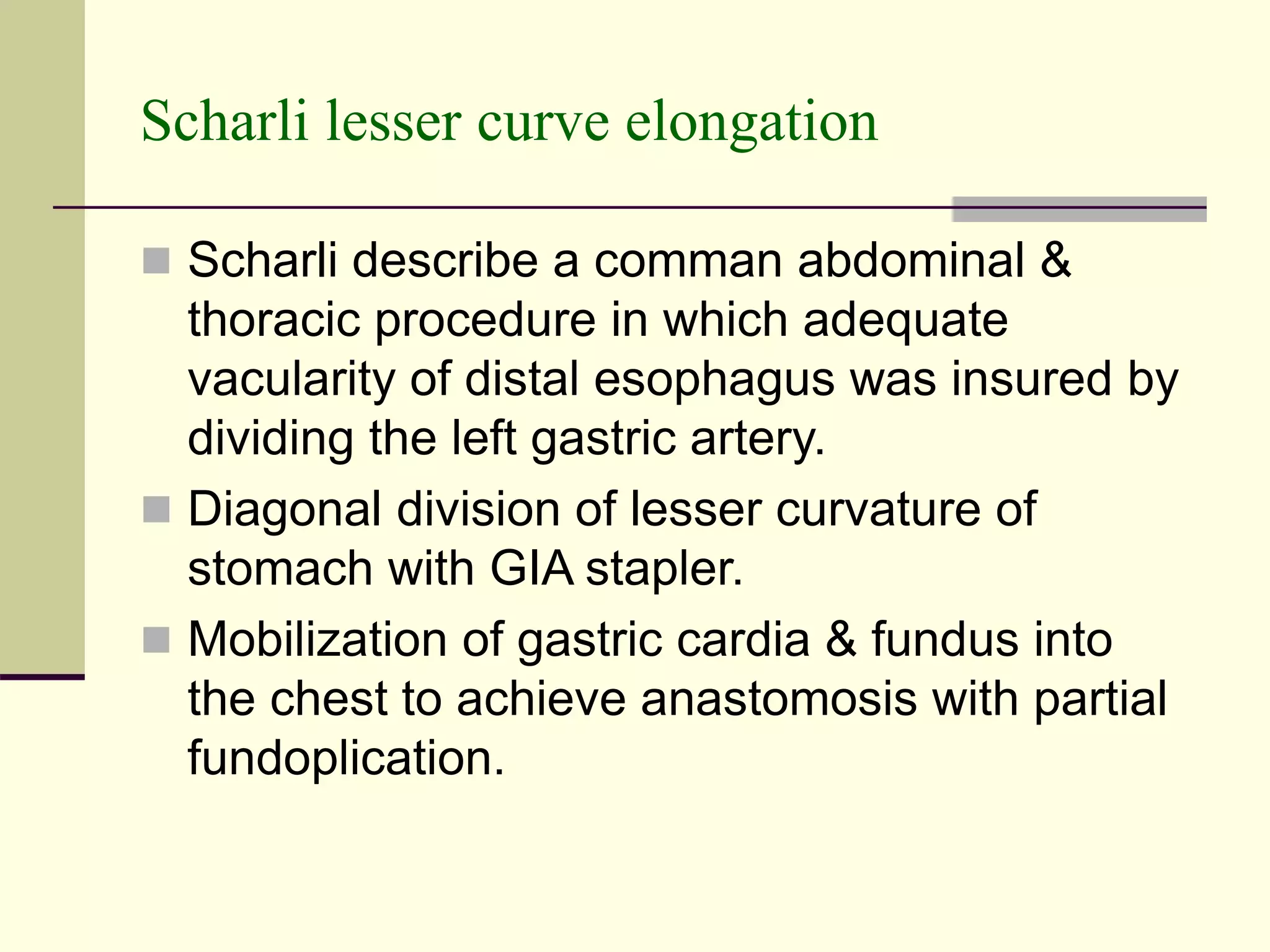
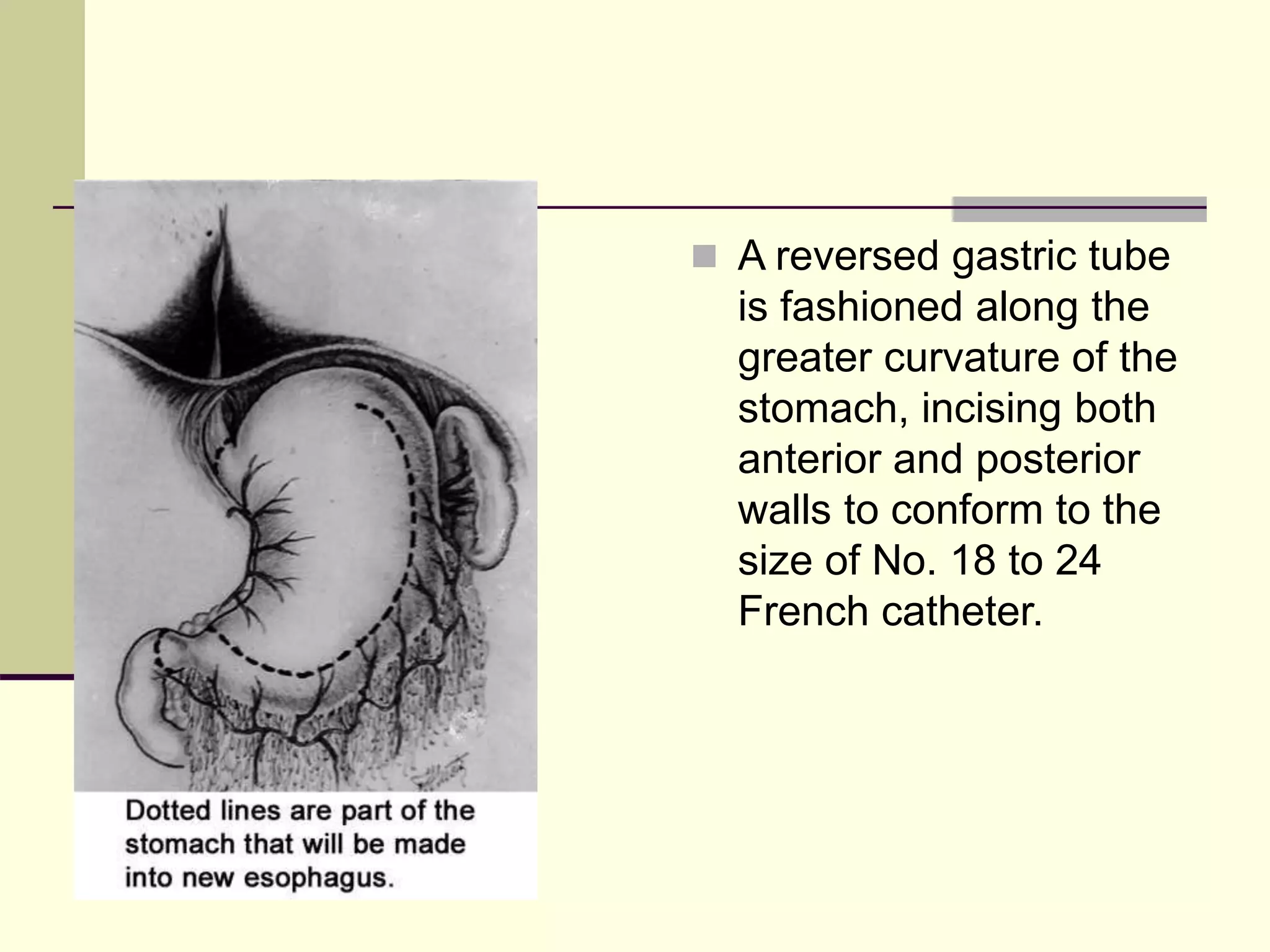
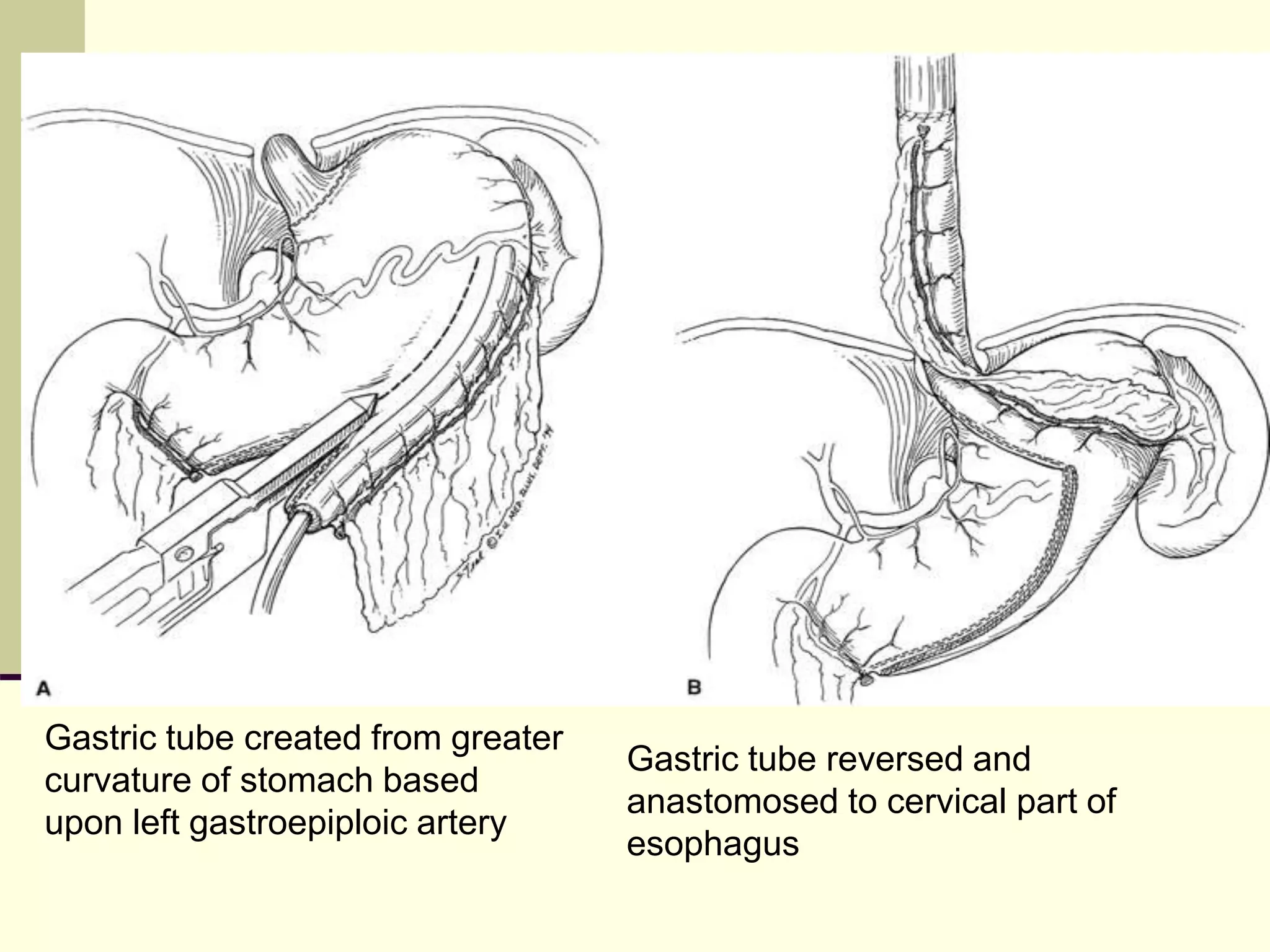
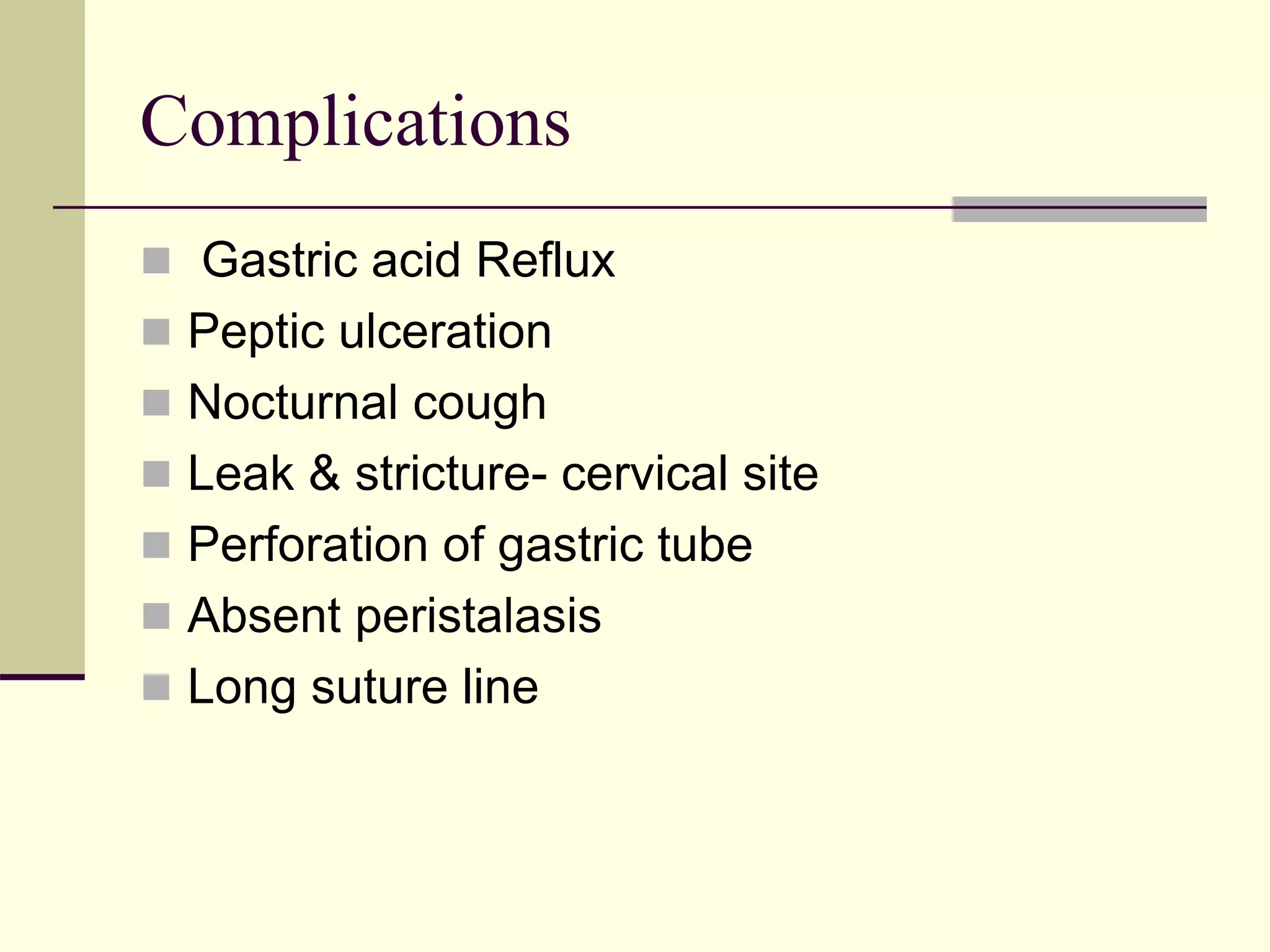
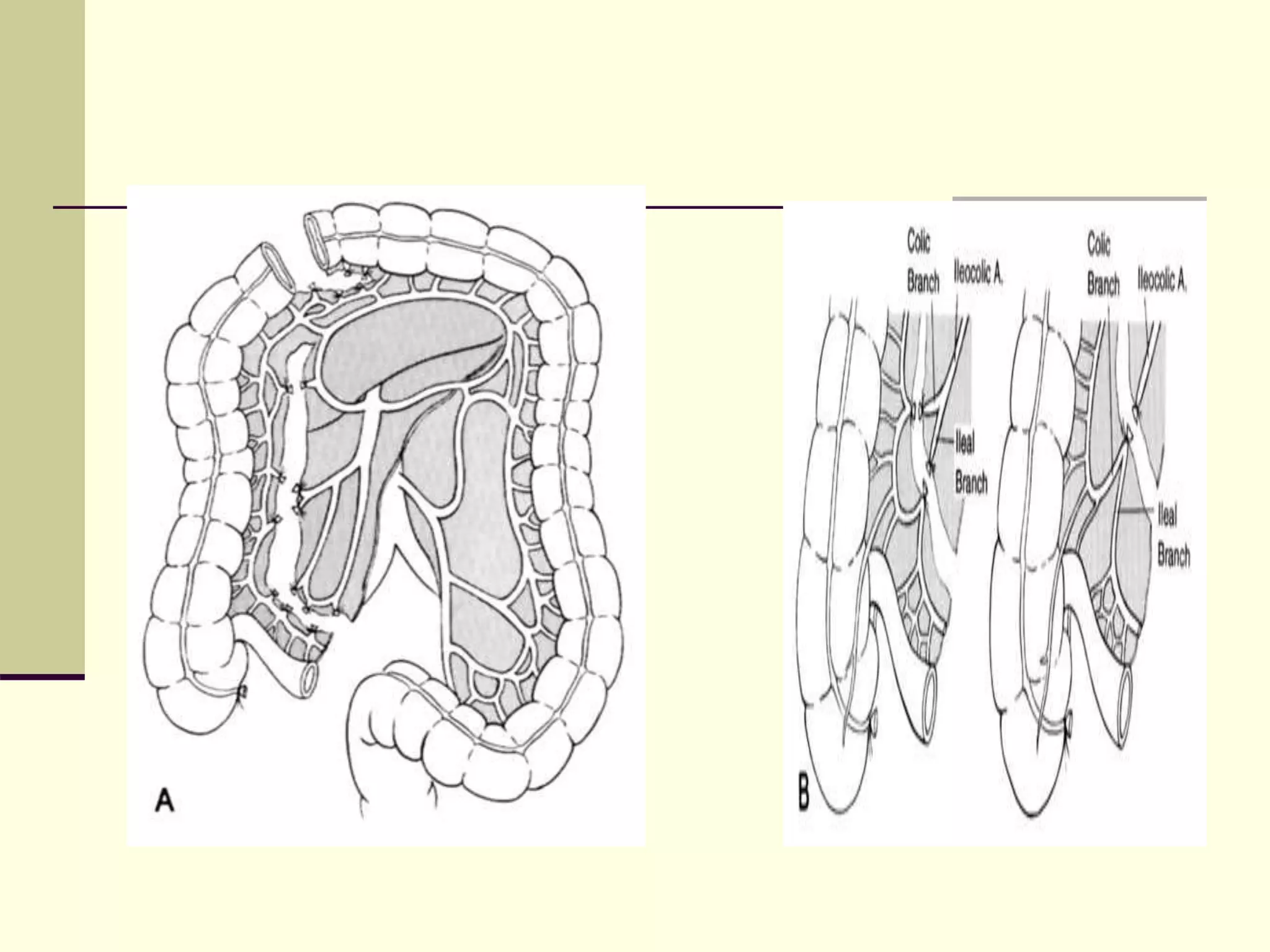
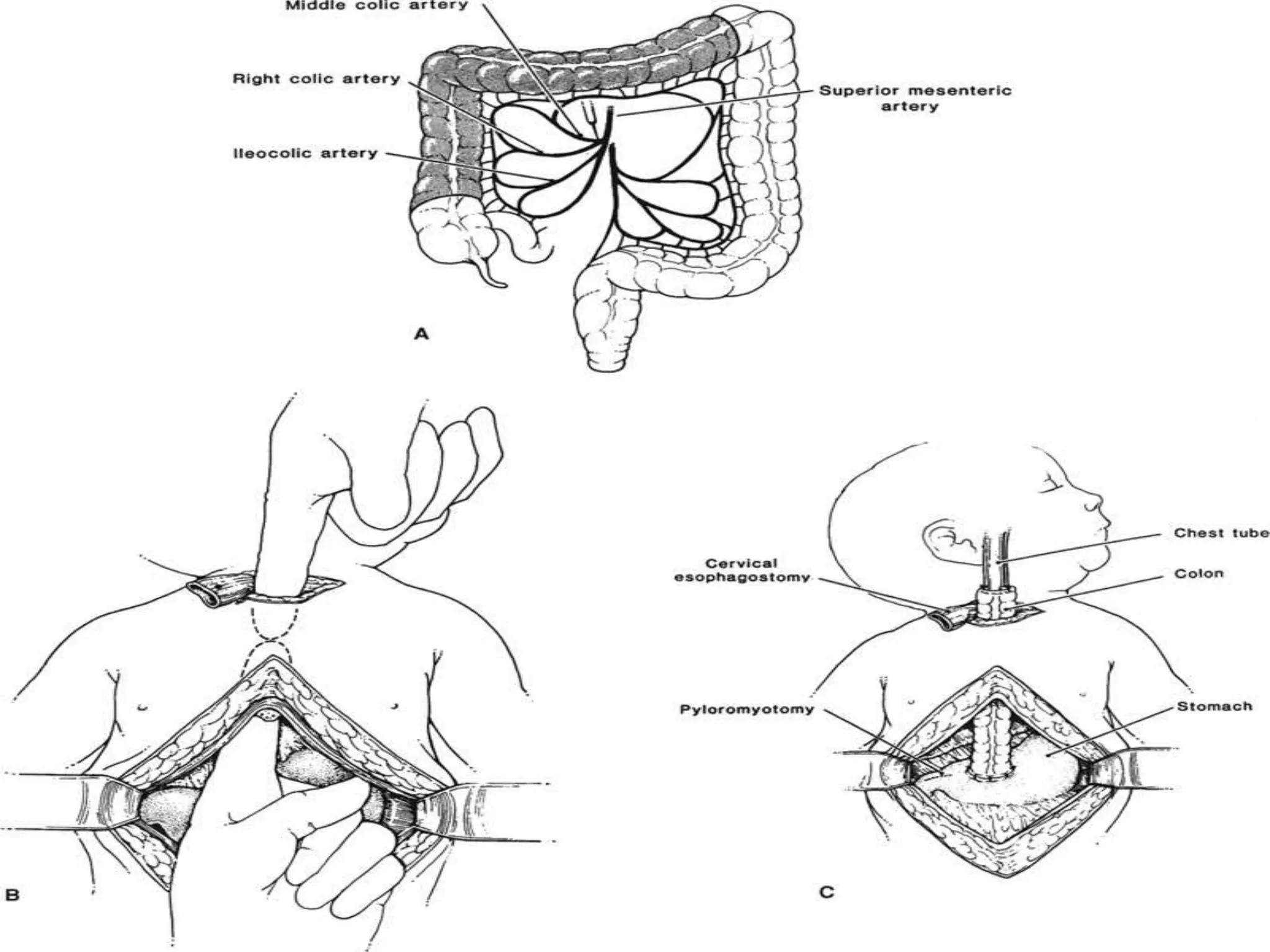
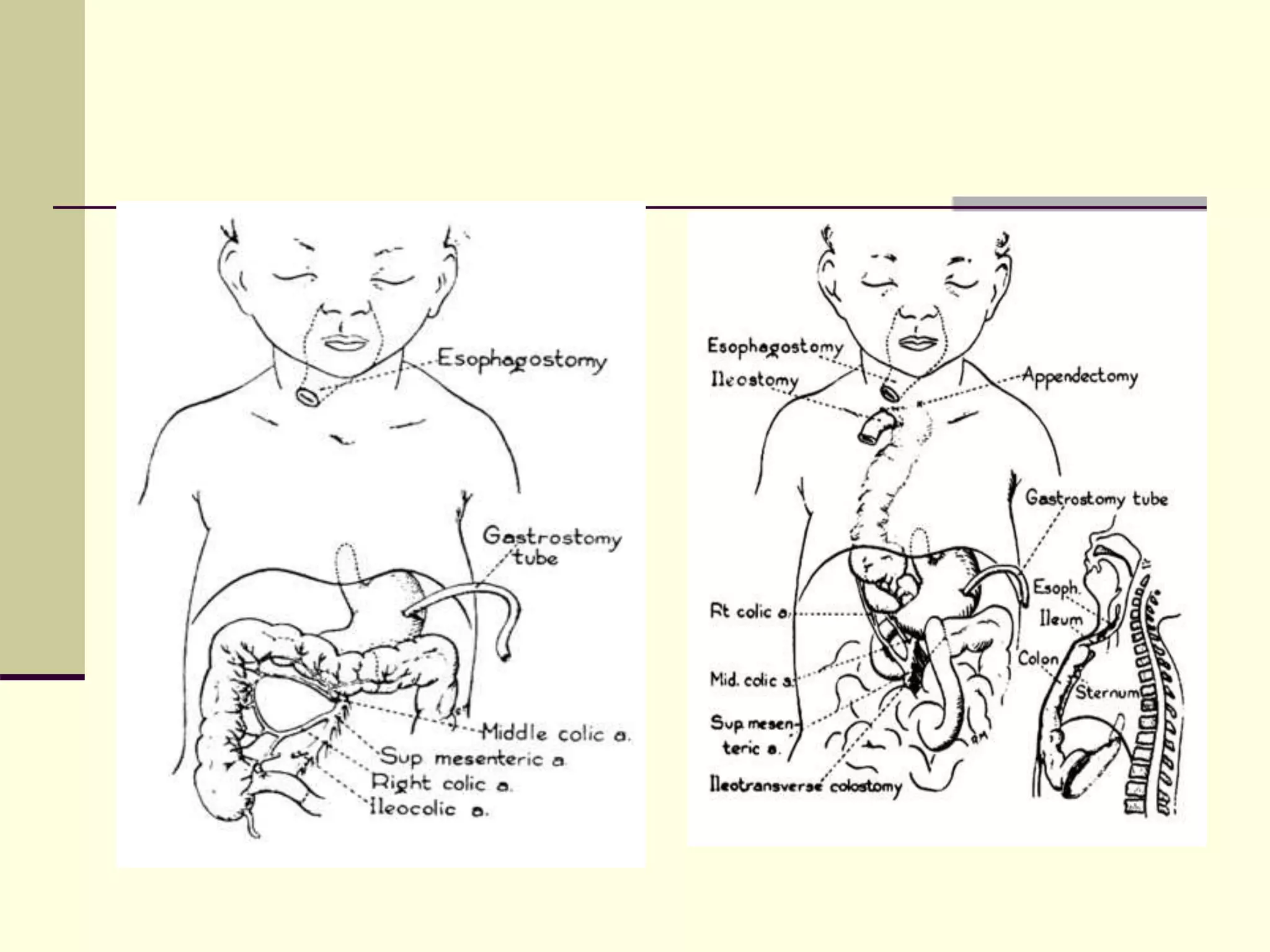
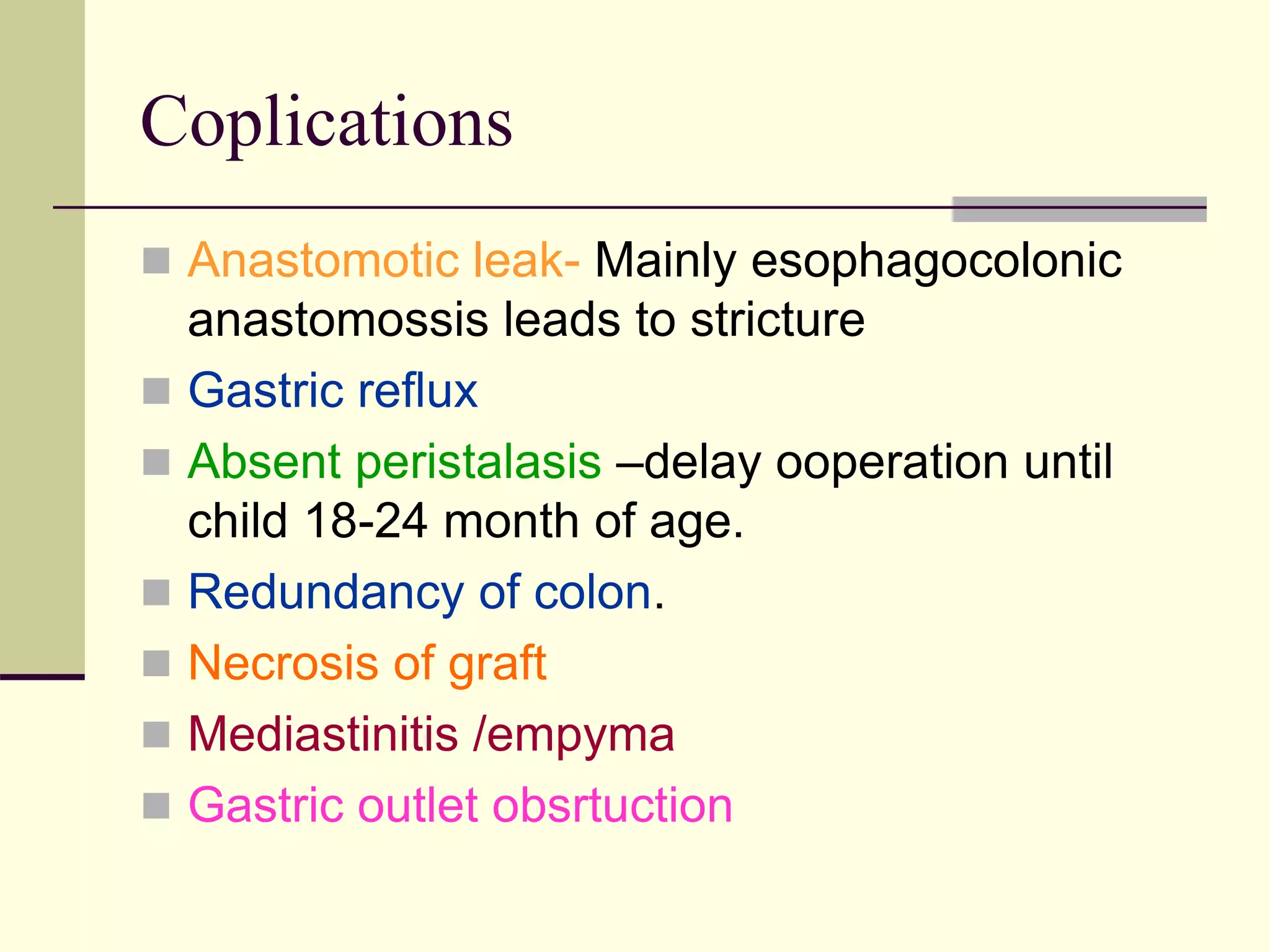
This document discusses tracheo-esophageal fistula (TEF), including its history, epidemiology, embryology, anatomy, classification, associated anomalies, symptoms, diagnosis, prognosis, preoperative management, and surgical repair. Key points include:
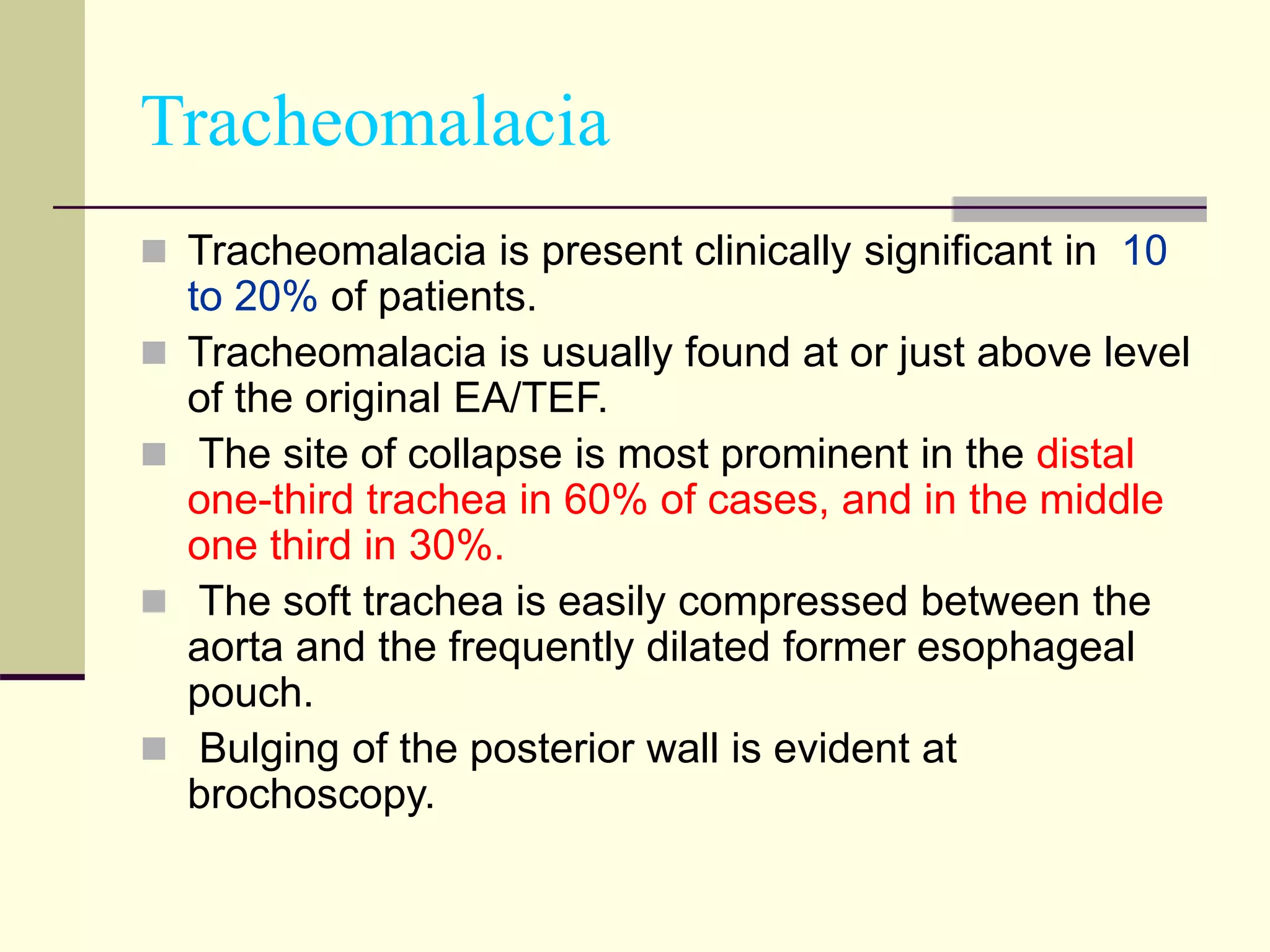
- TEF is an abnormal connection between the esophagus and trachea that often occurs with esophageal atresia.
- It has no known cause but risk factors include certain medications in pregnancy and maternal diabetes.
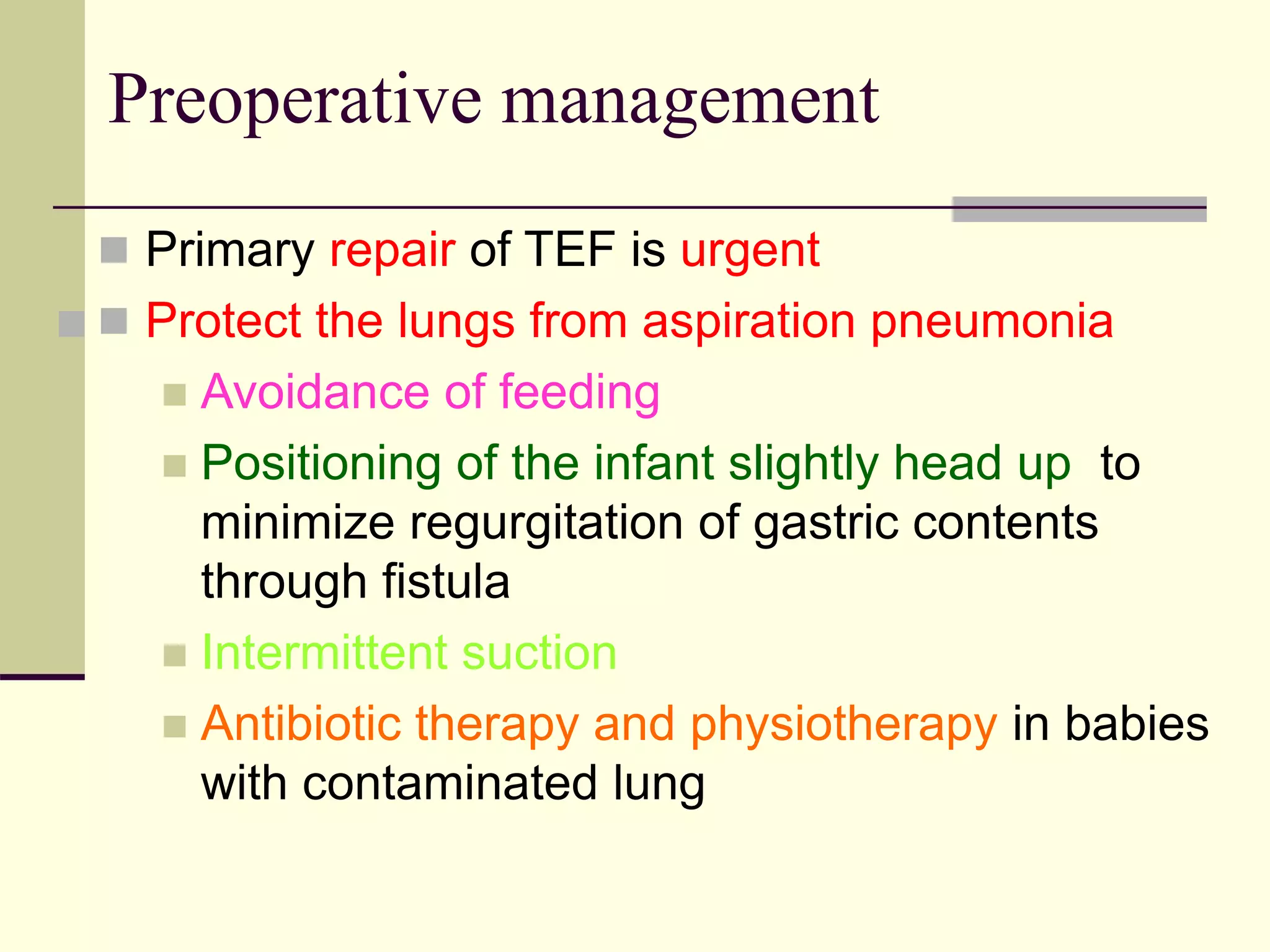
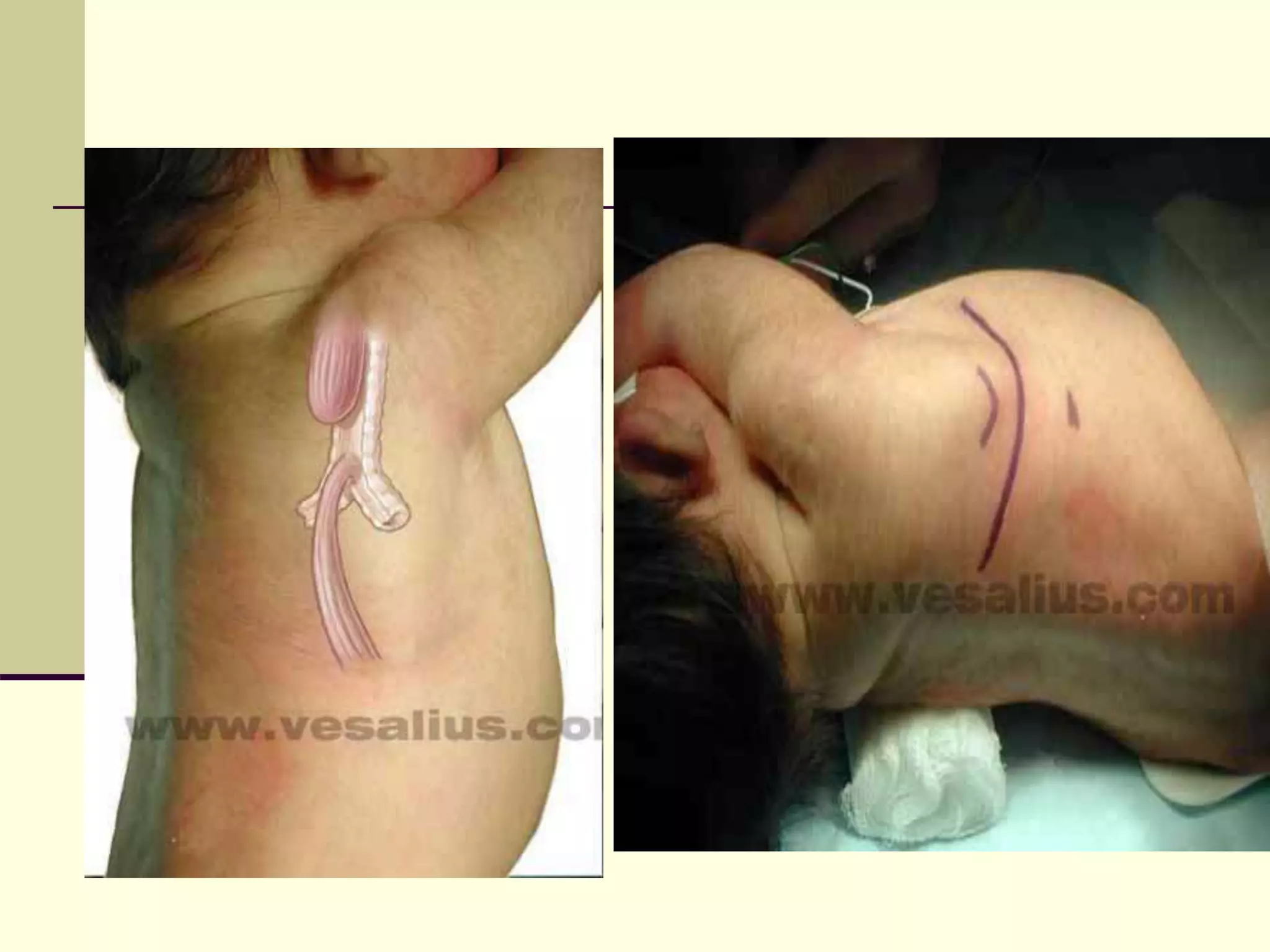
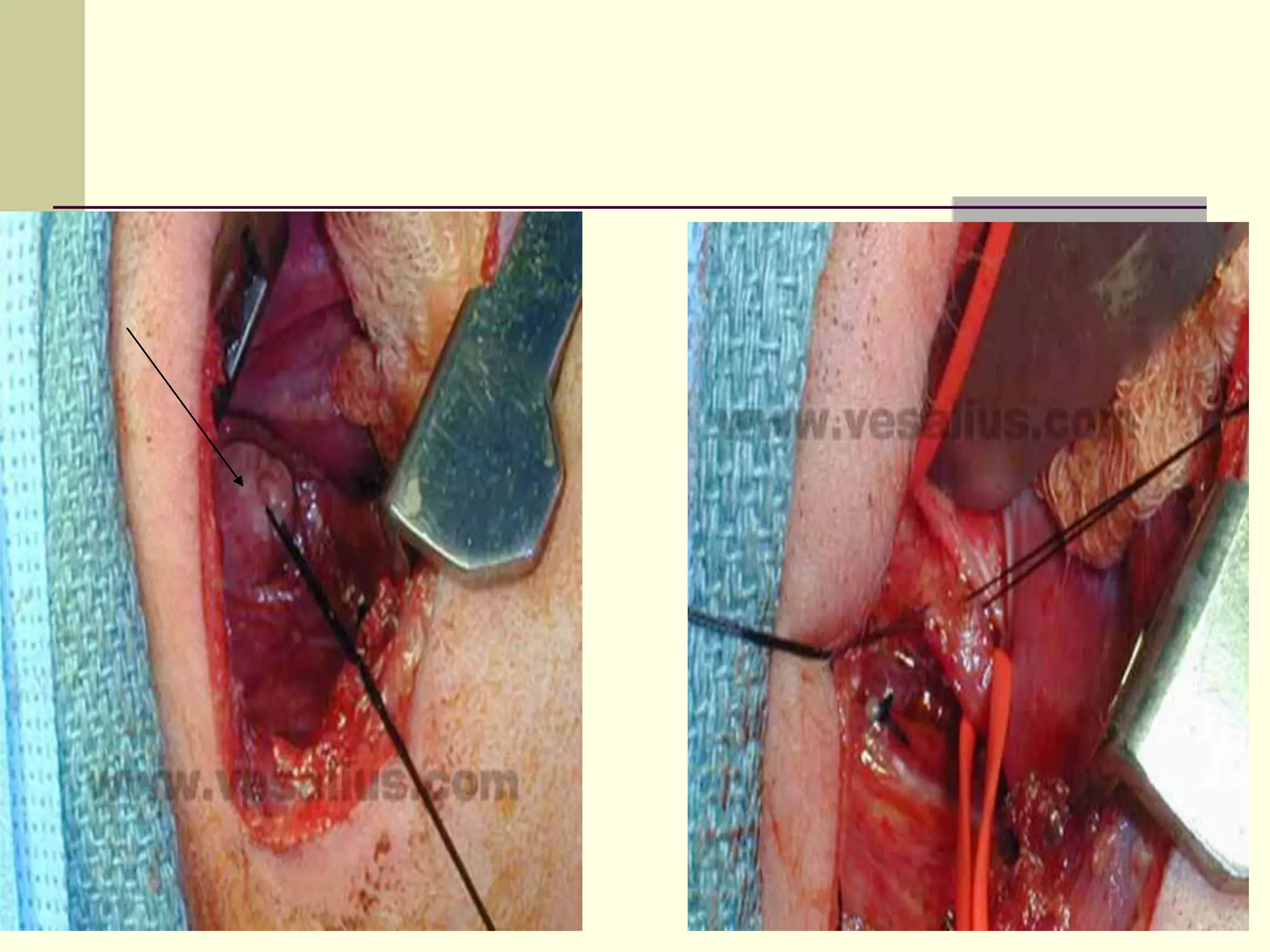
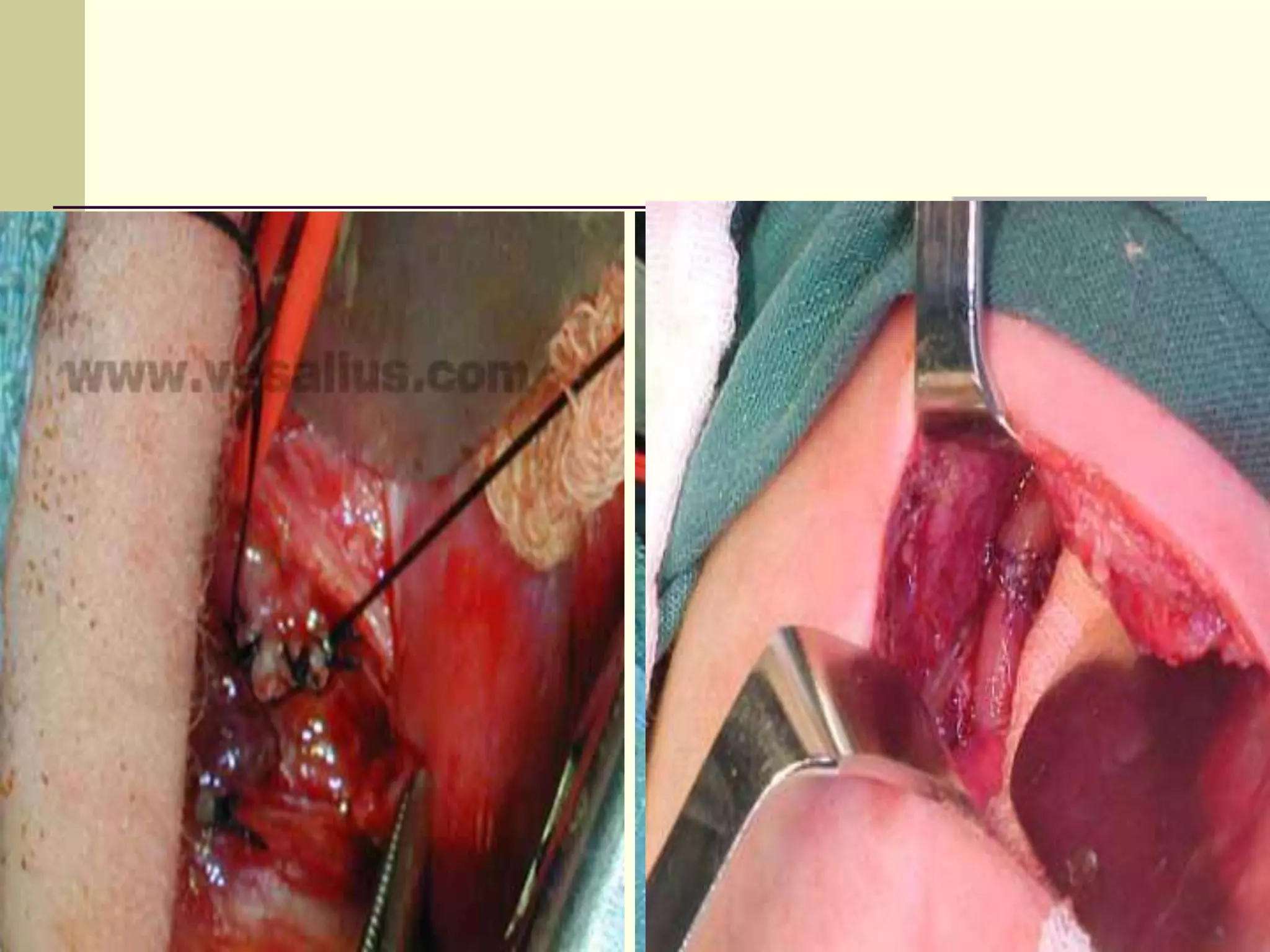
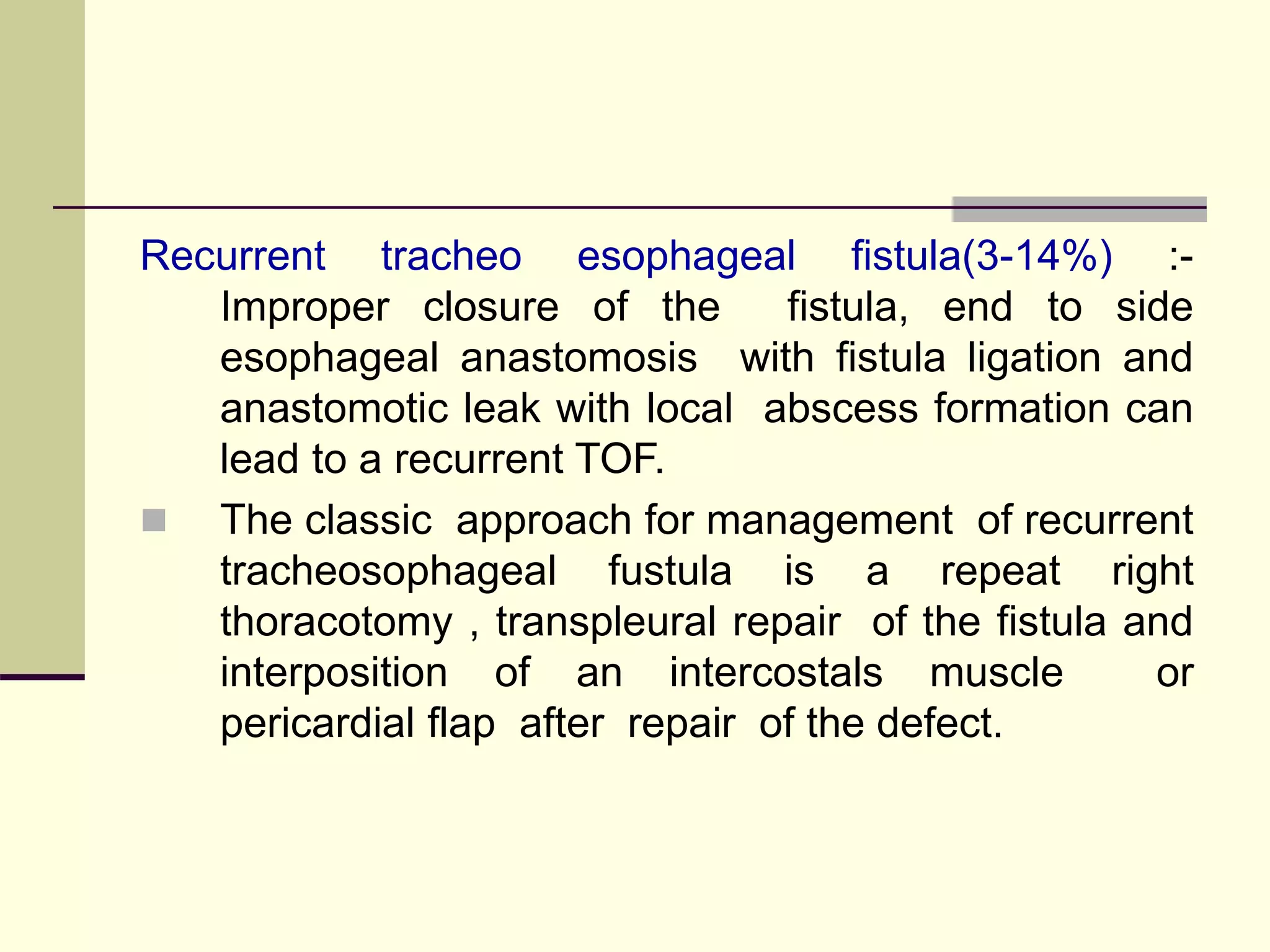
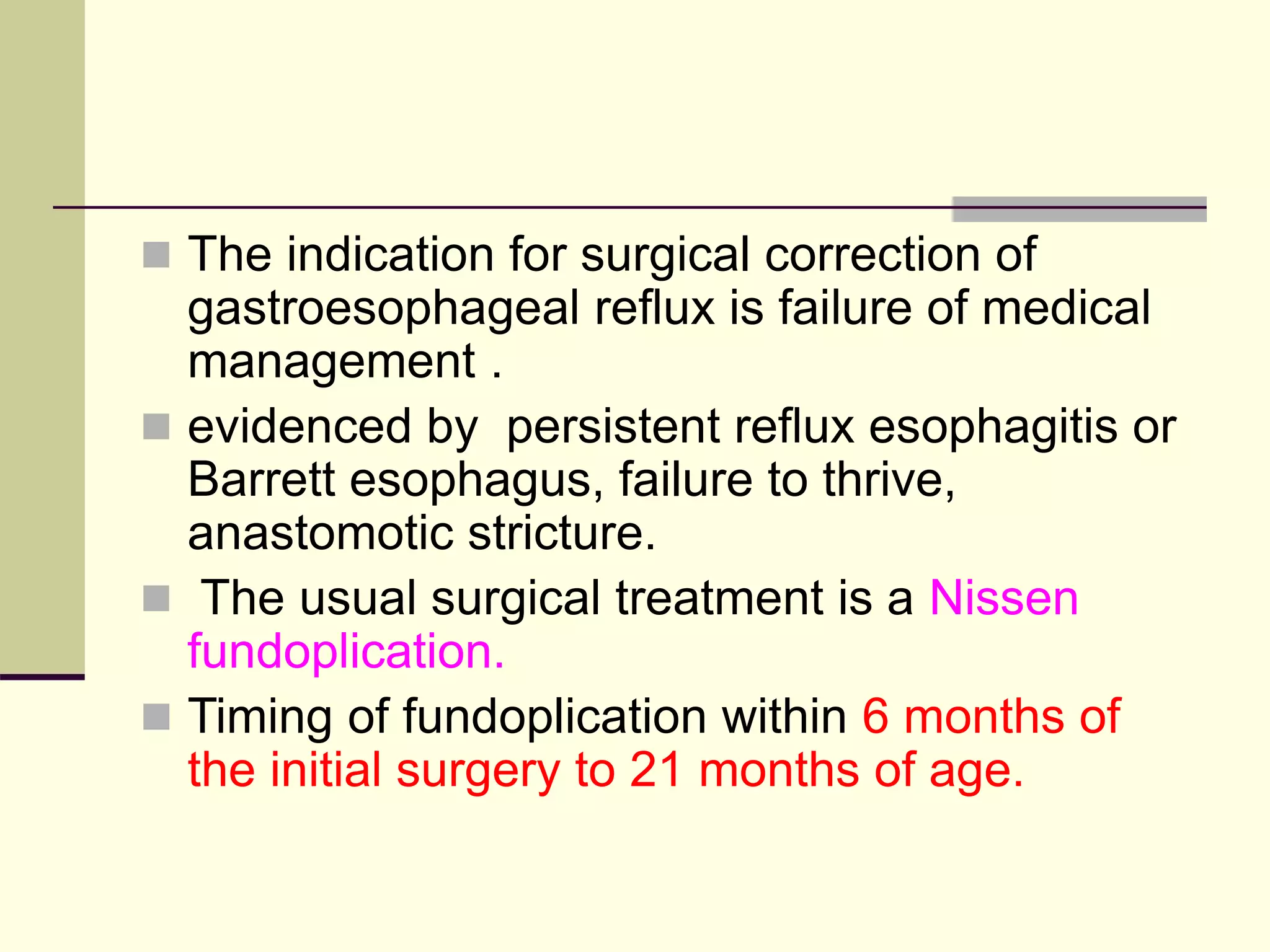
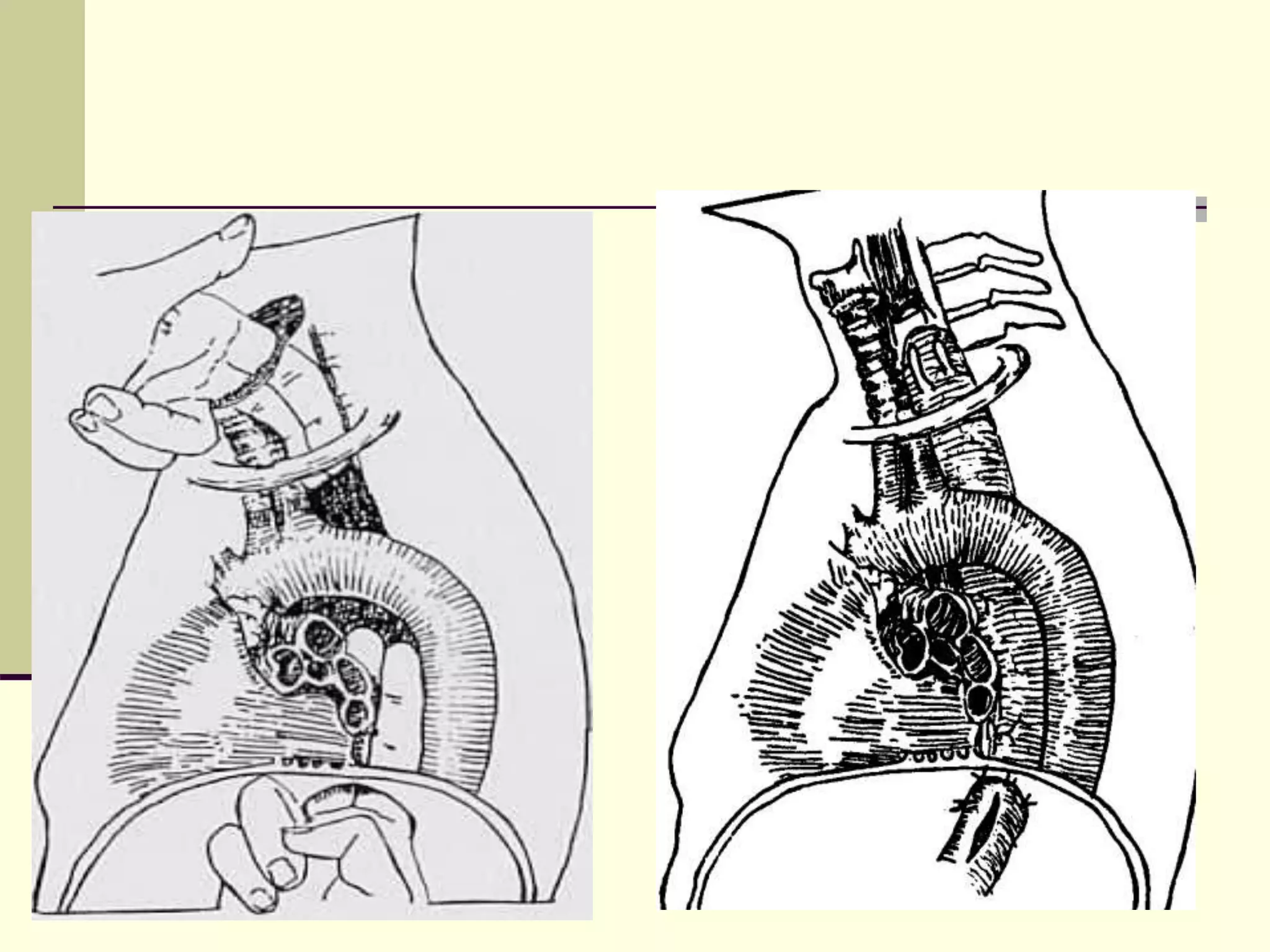
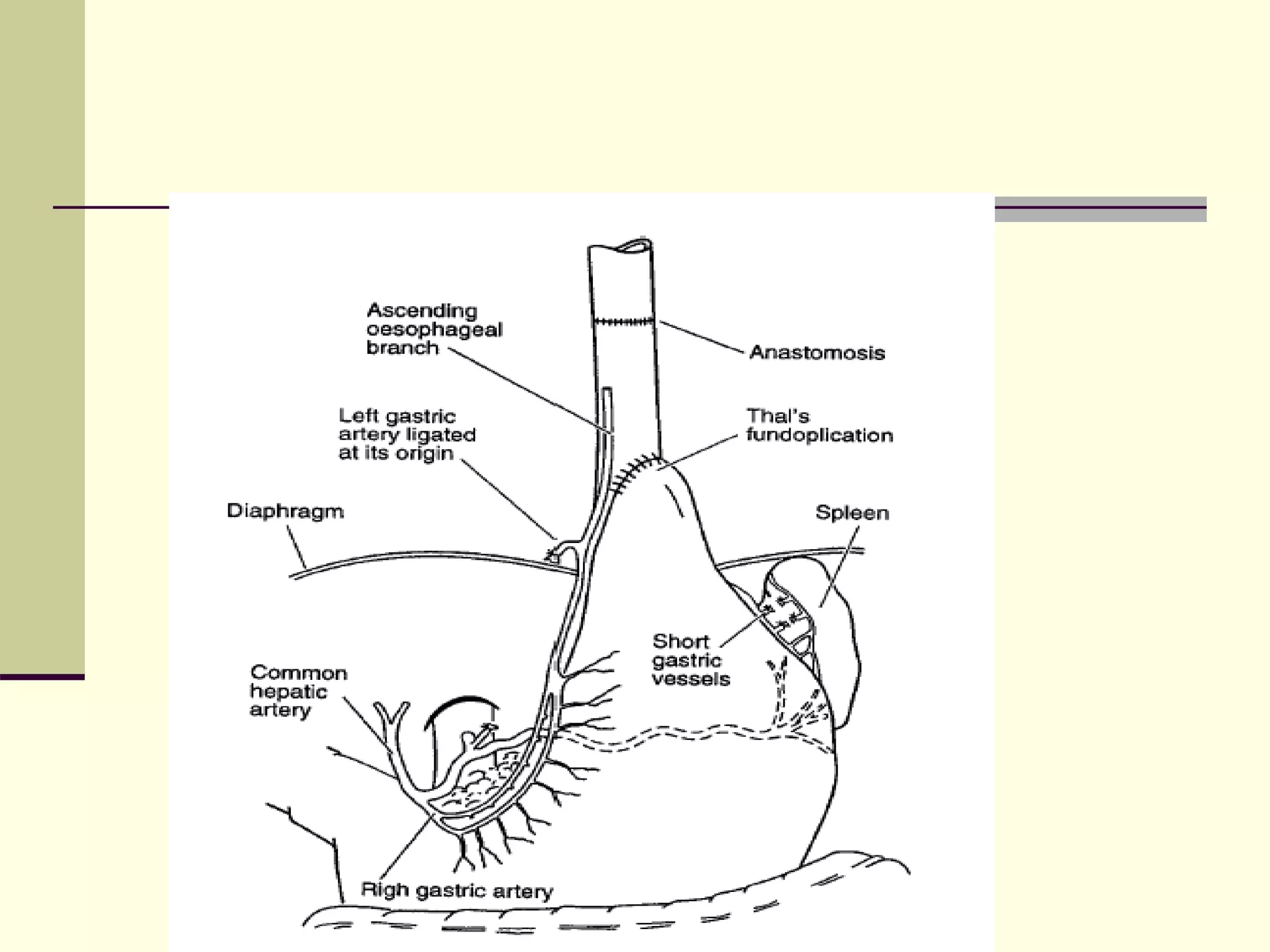
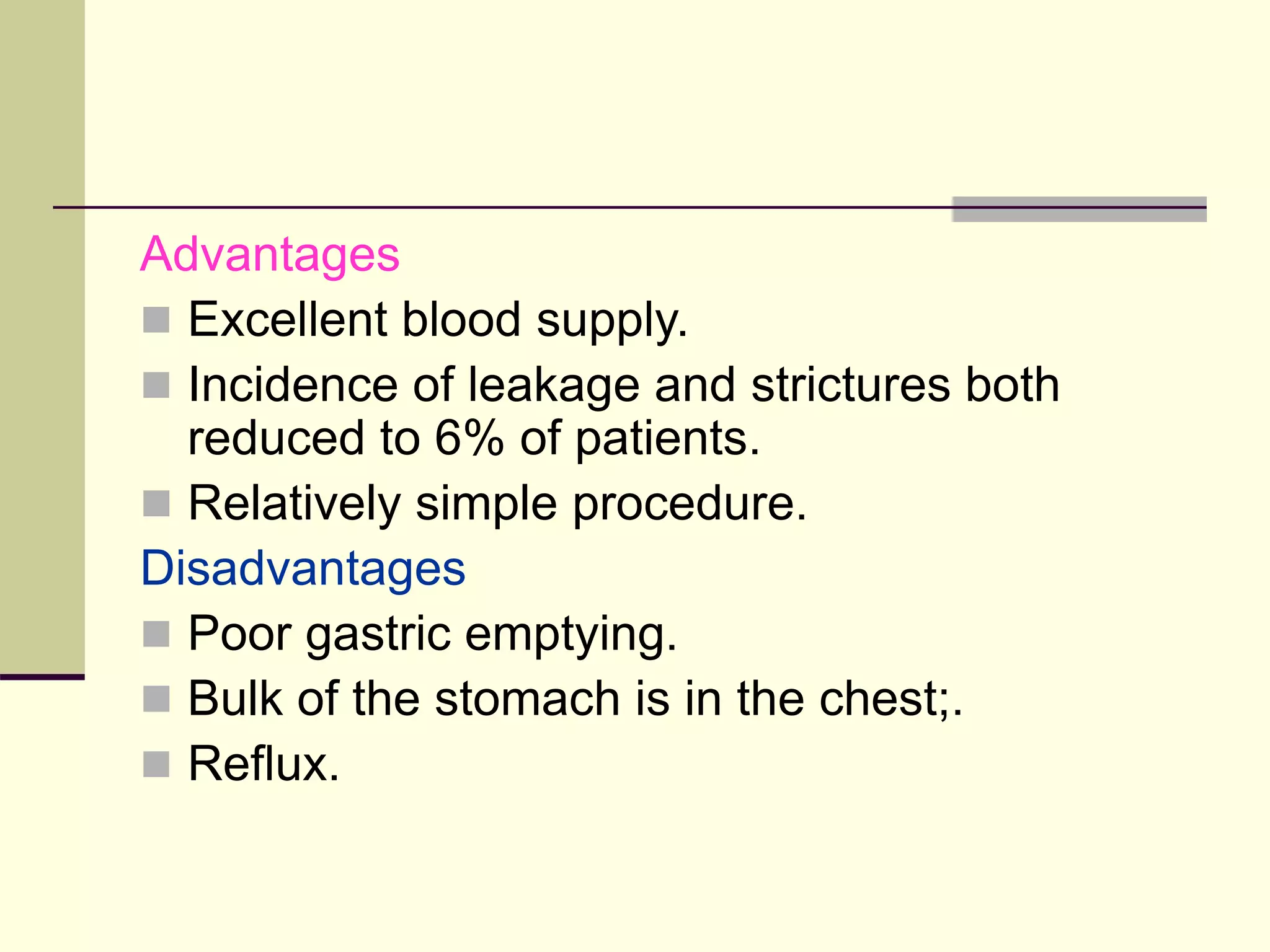
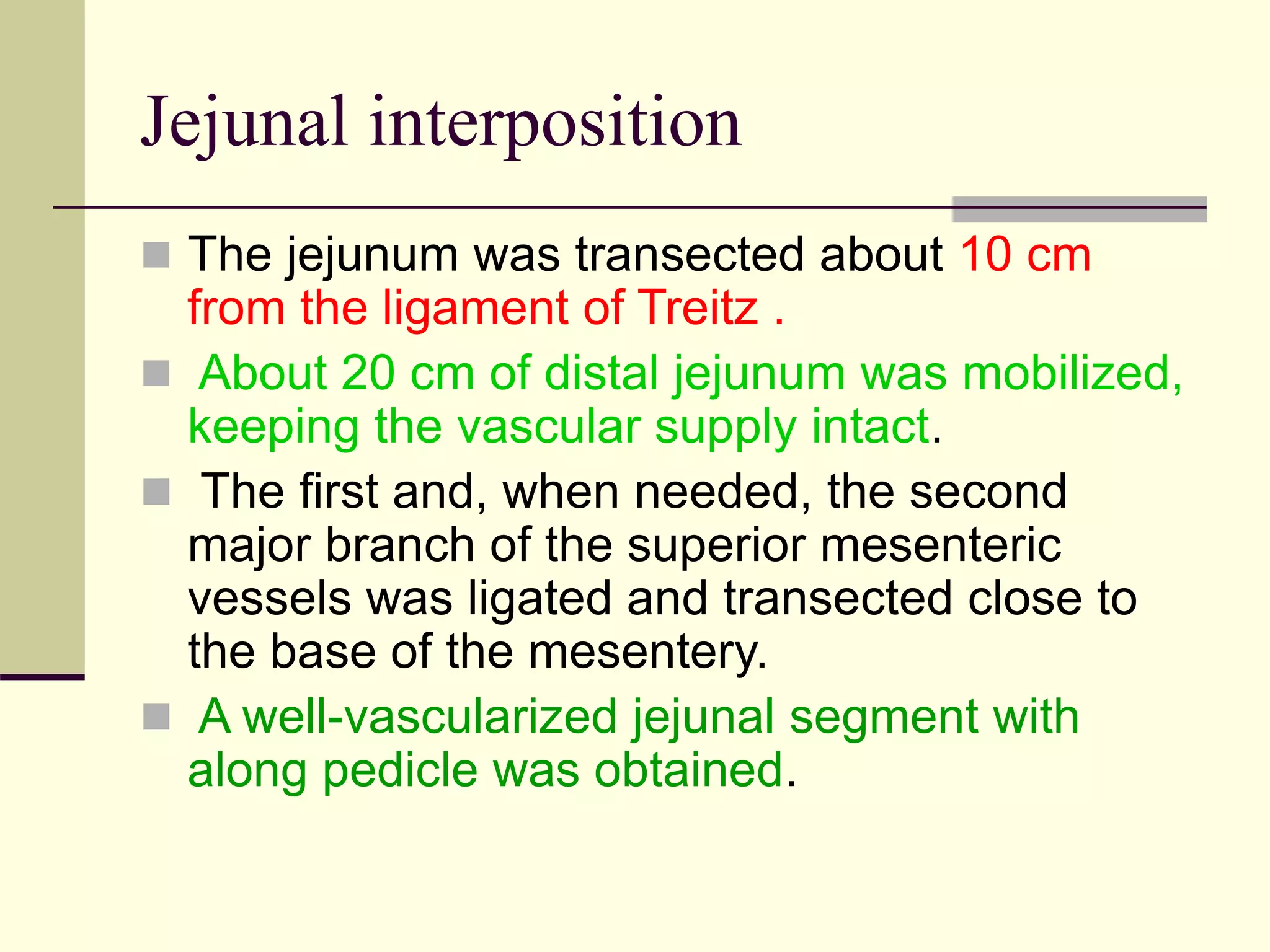
- Surgical repair via thoracotomy aims to ligate the fistula and anastomose the esophageal ends in a single stage for optimal outcomes.
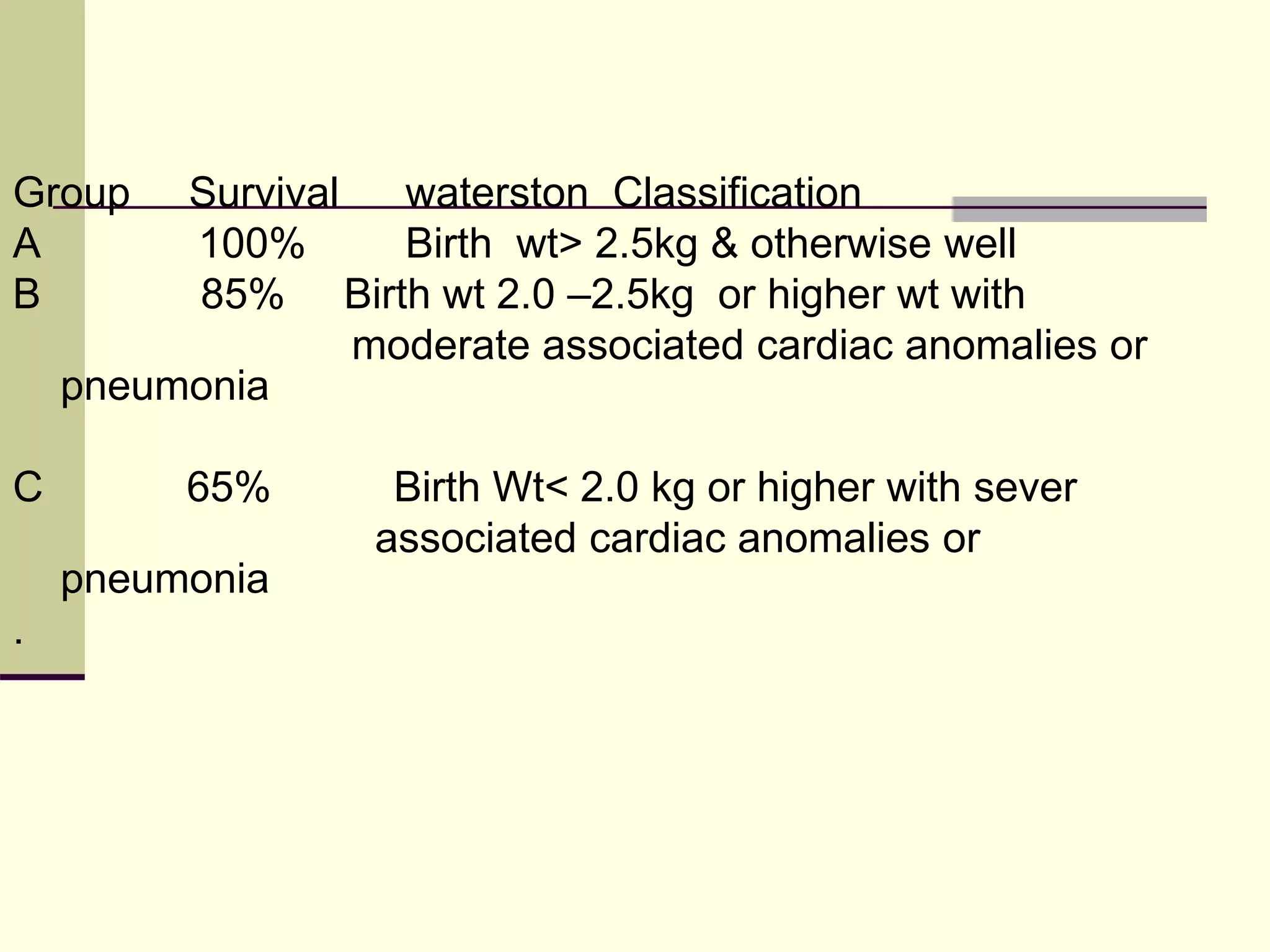
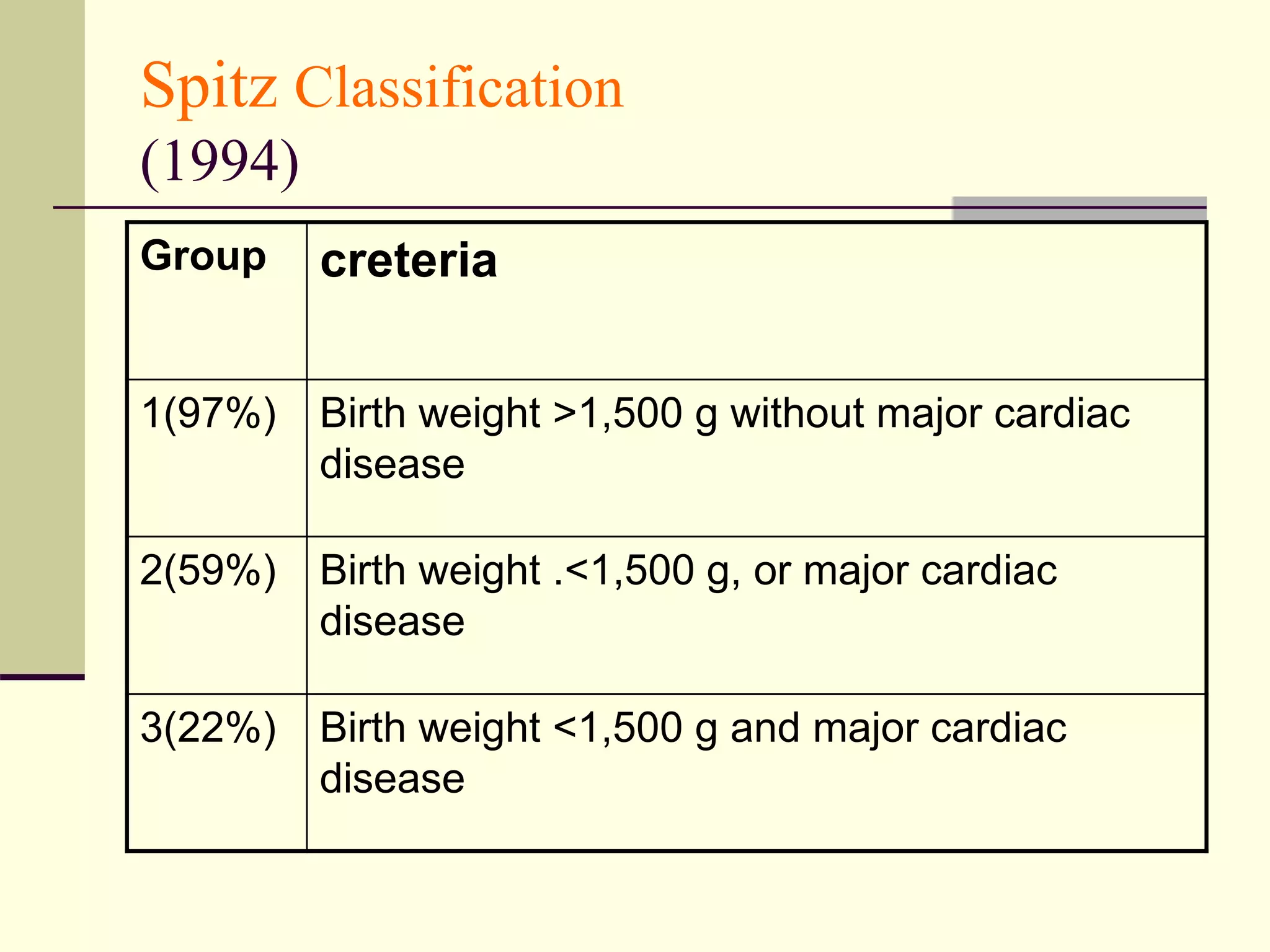
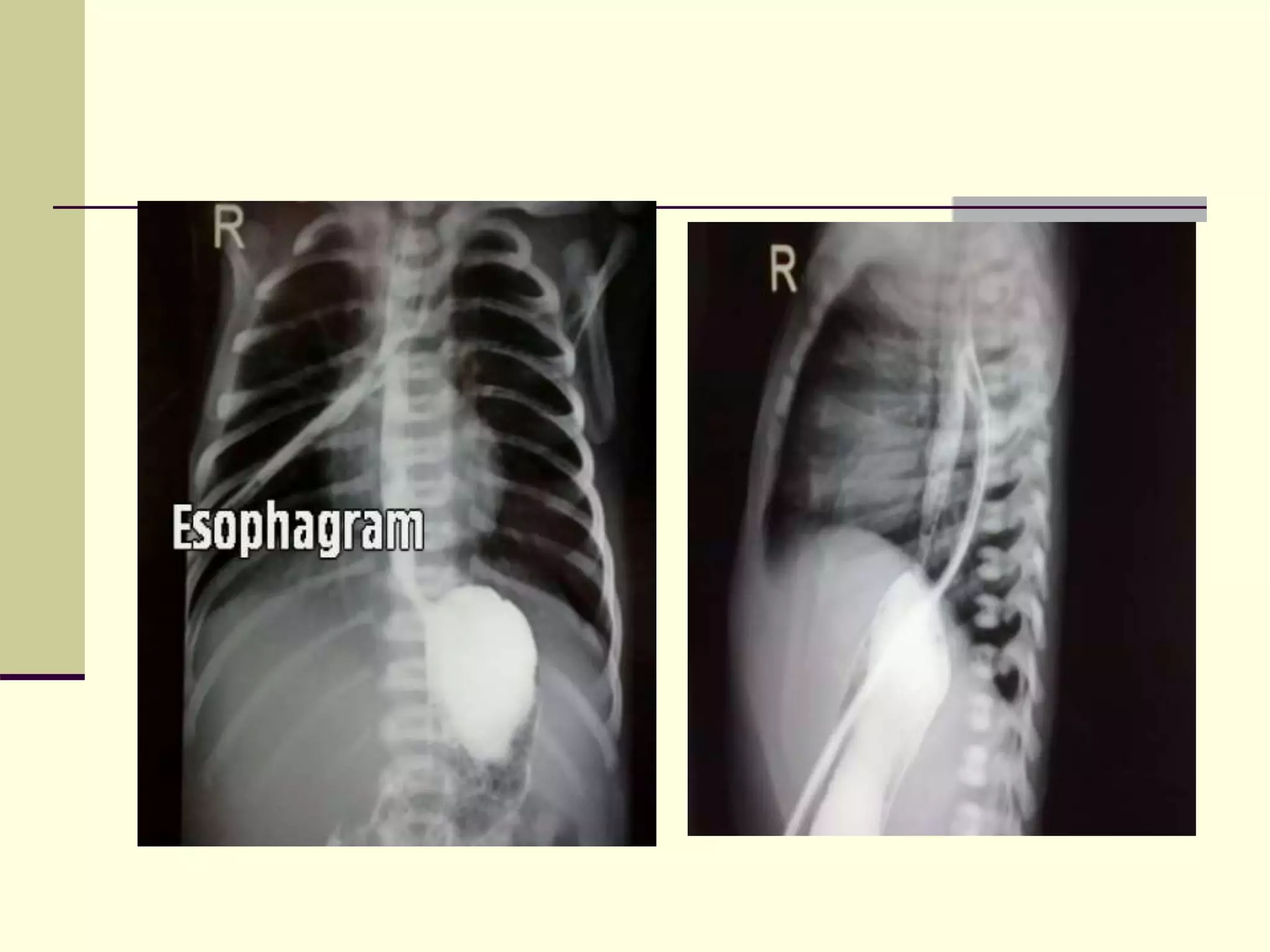
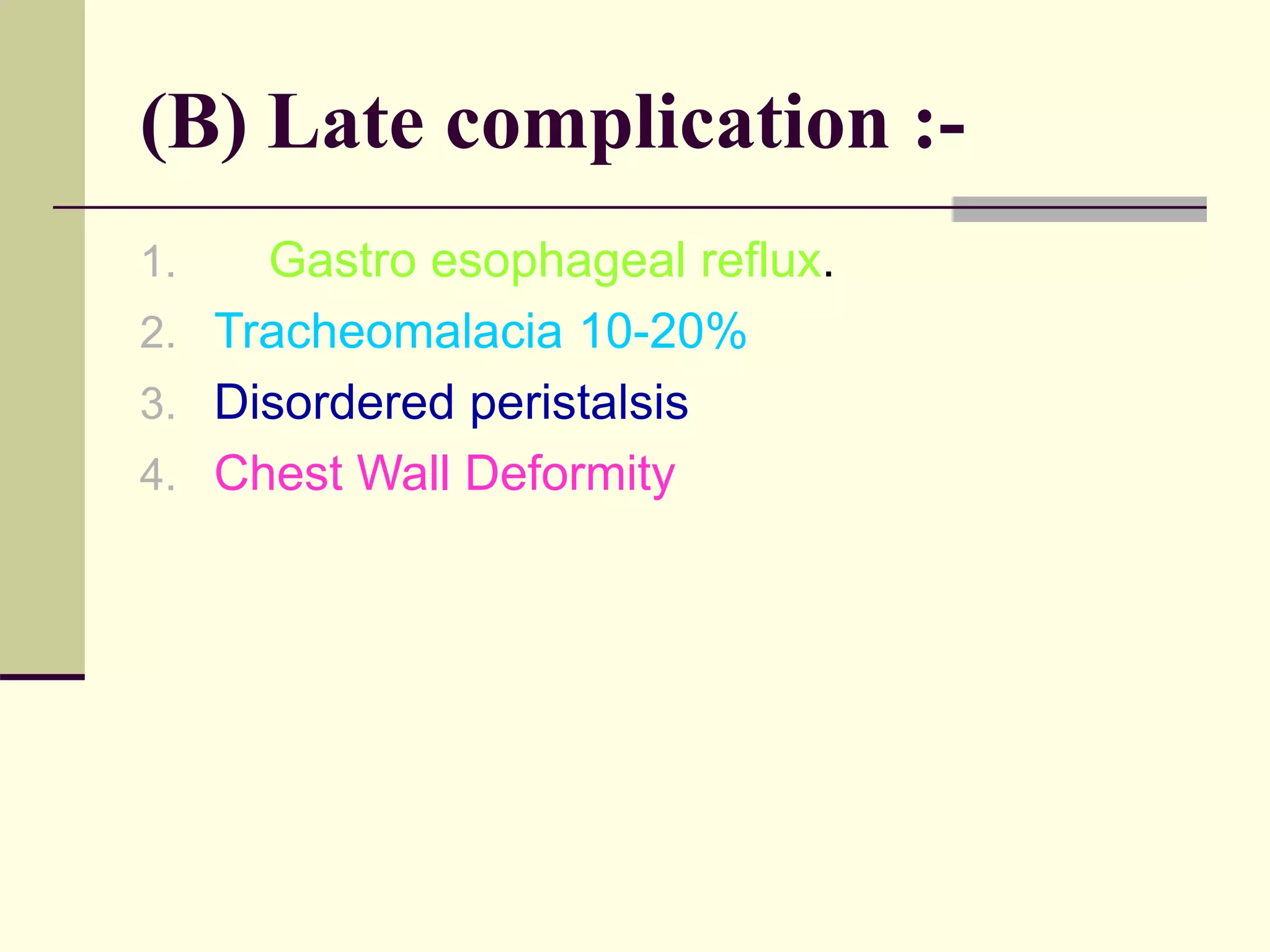
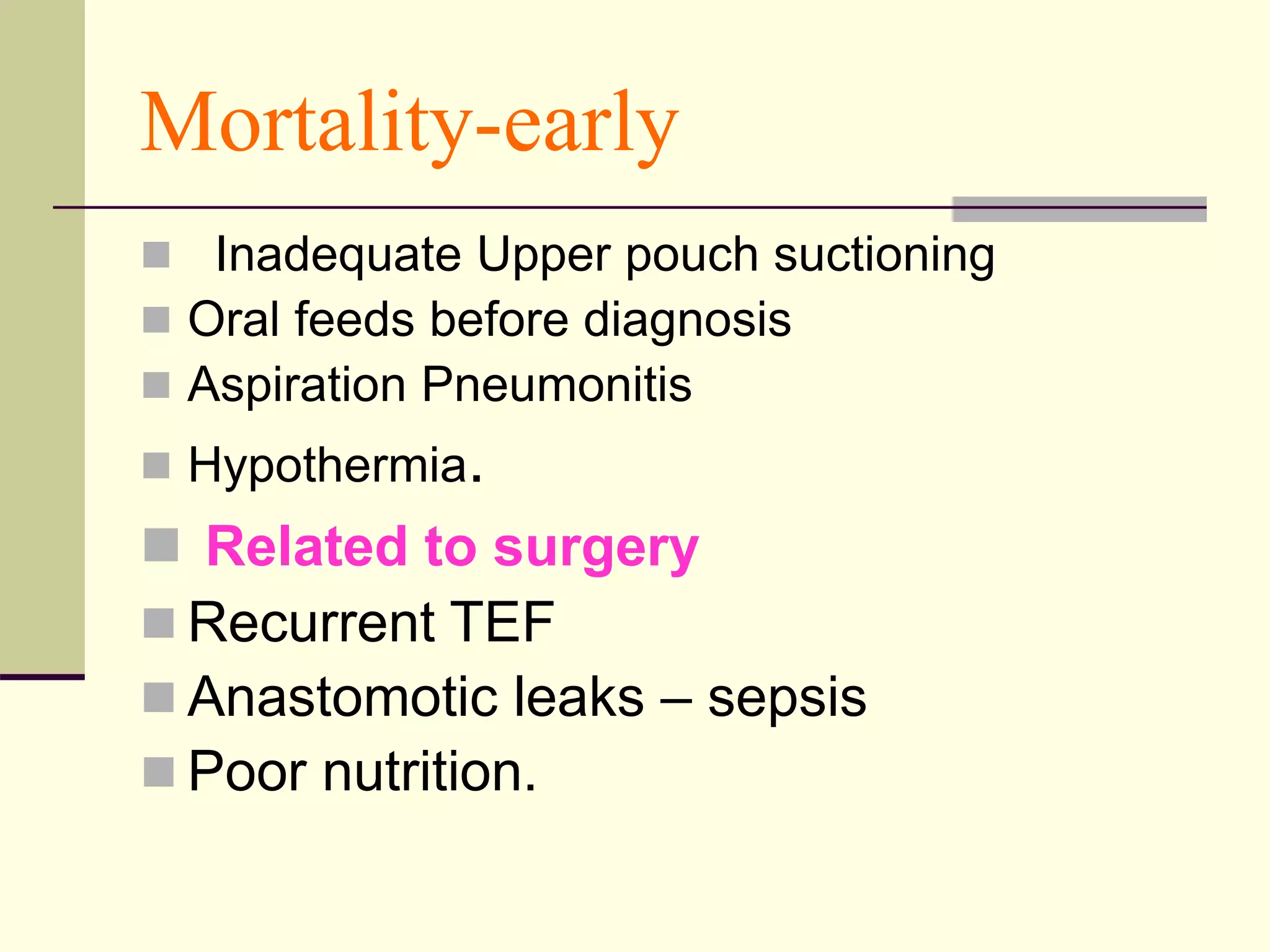
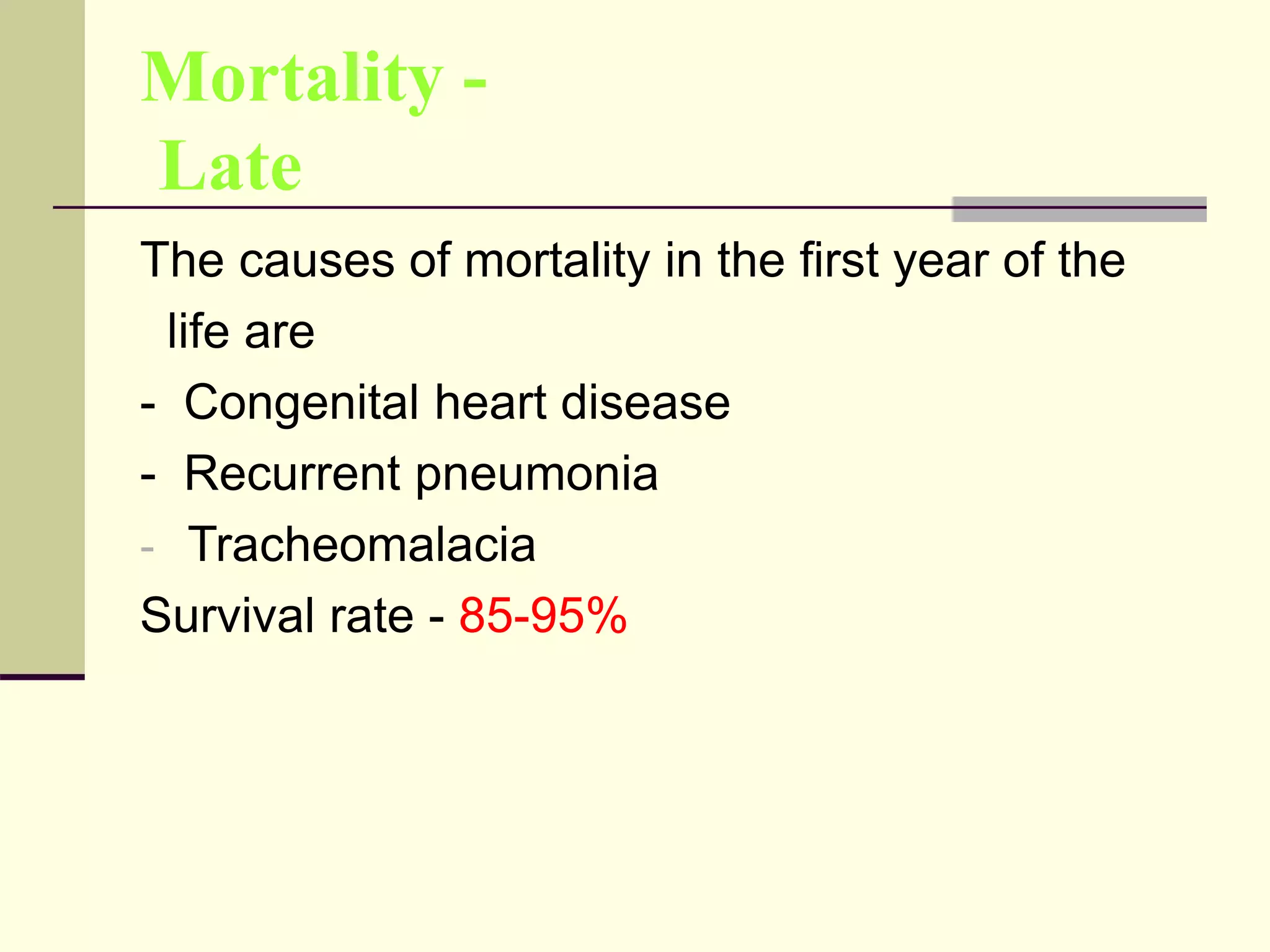
- Prognosis depends on factors like birth weight and presence of cardiac defects.