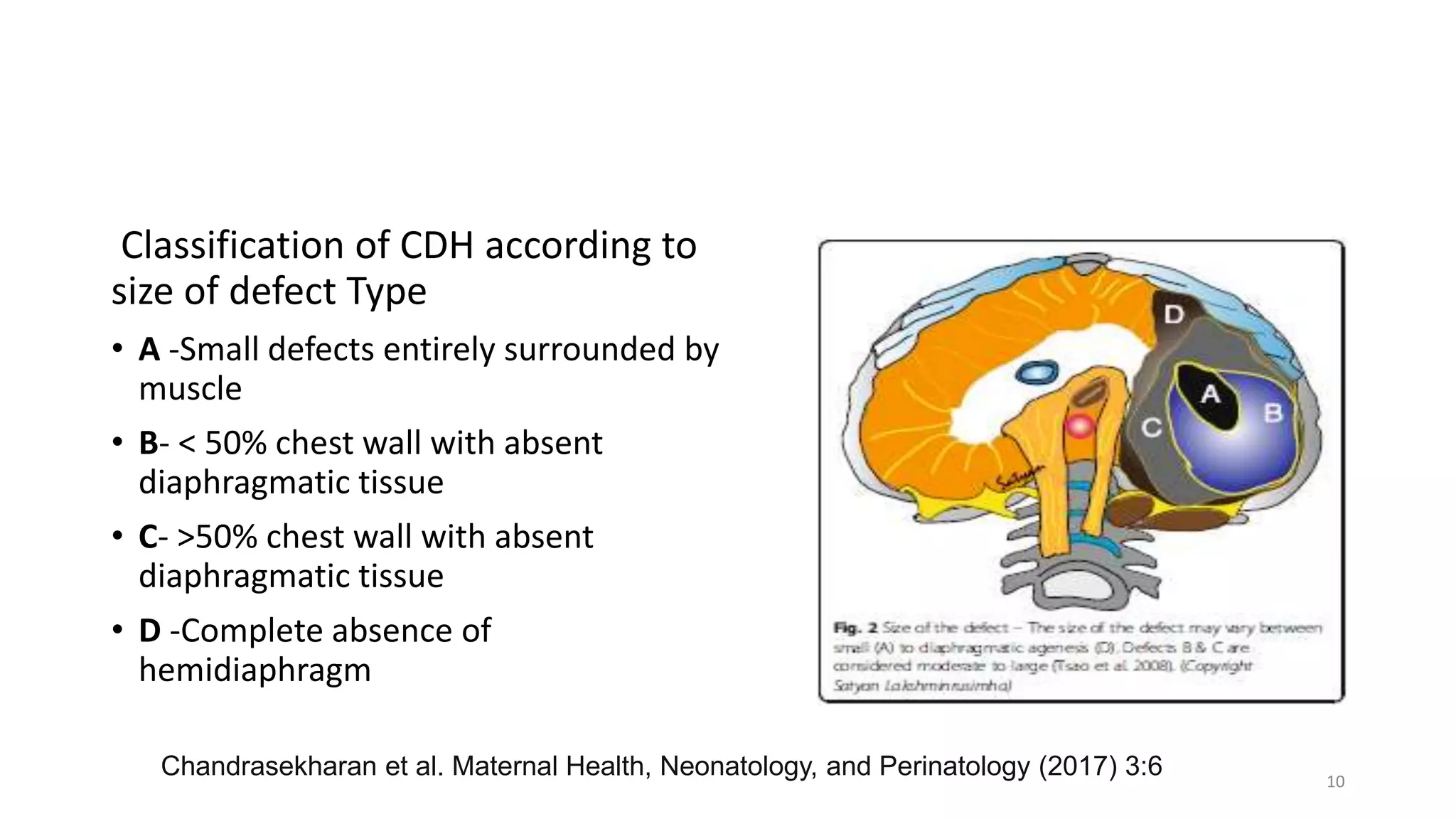
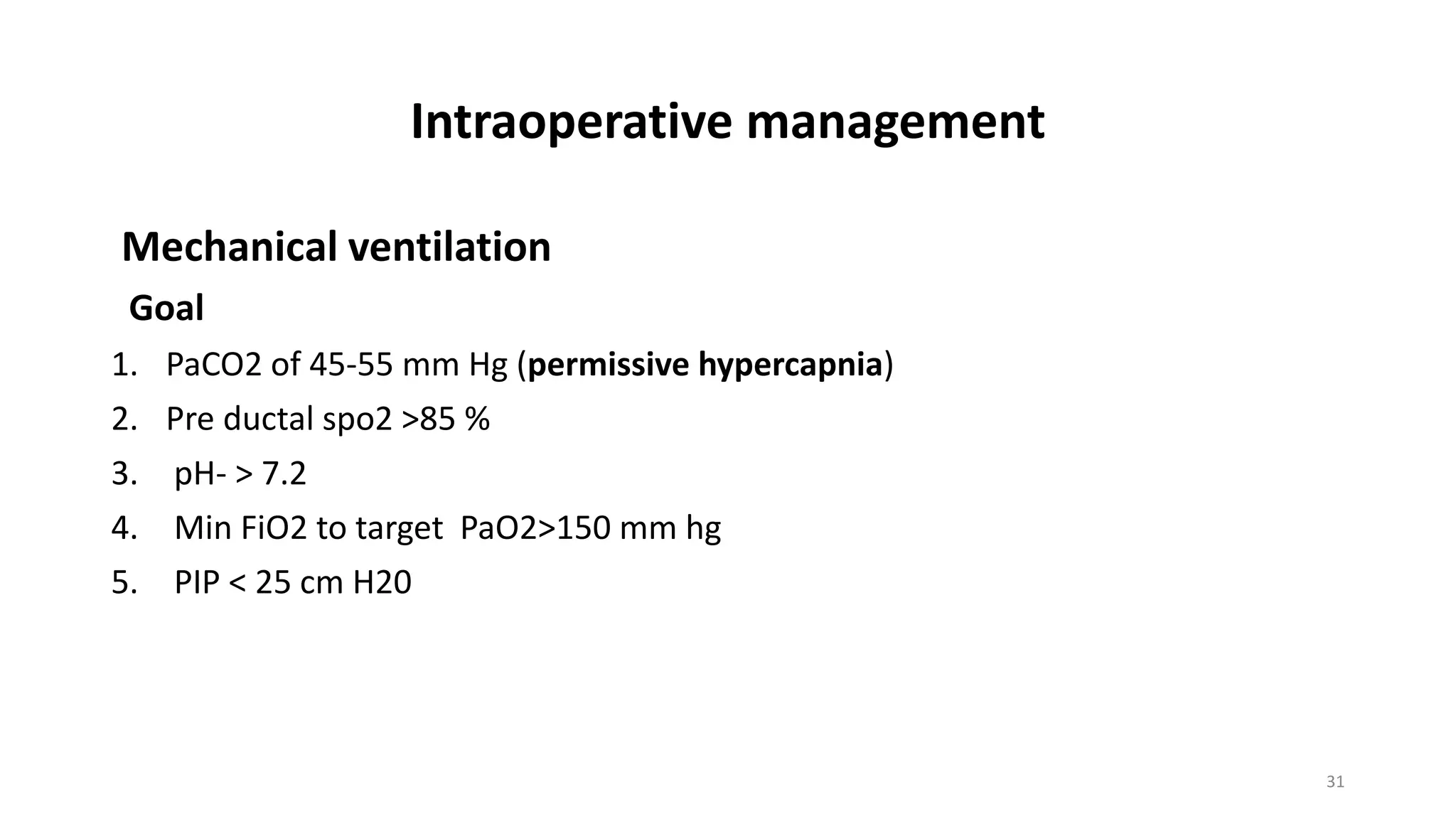
This document discusses congenital diaphragmatic hernia (CDH), a birth defect where organs protrude into the chest cavity due to a hole in the diaphragm. It covers the embryology, pathophysiology, diagnosis, and management of CDH. Surgical repair is the only treatment, but stabilization of the patient's respiratory and general status is needed first. Extracorporeal membrane oxygenation (ECMO) has improved survival for CDH. Long-term follow up is also important due to potential complications. A regional anesthesia method without opioids allowed early operating room extubation for CDH repair in one study.