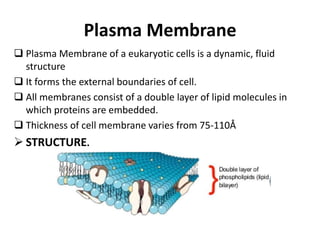
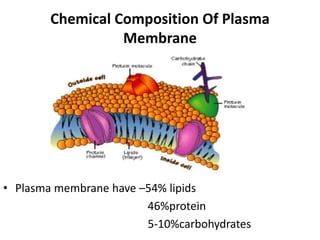
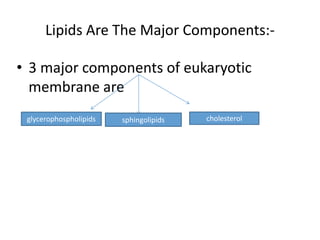
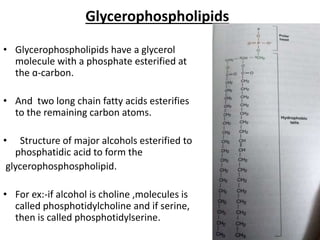
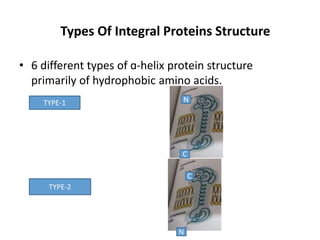
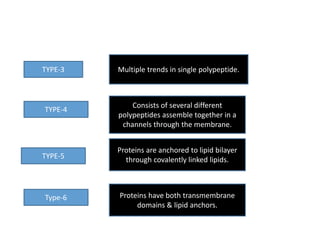
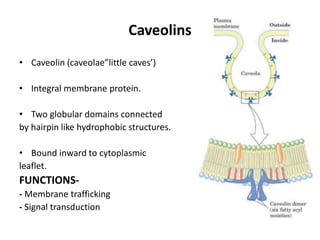
The document discusses the biological membrane and its chemical composition. It notes that the plasma membrane is the outer boundary of cells, consisting of a double layer of lipid molecules with embedded proteins. The major components of membranes are glycerophospholipids, sphingolipids, and cholesterol. Glycerophospholipids are amphipathic lipids that form the lipid bilayer structure. The fluid mosaic model describes membranes as a fluid structure with lipids and proteins able to move laterally. Membrane proteins can be integral or peripheral, and help with cell functions like transport and signaling. Membrane fluidity is influenced by temperature and lipid composition.