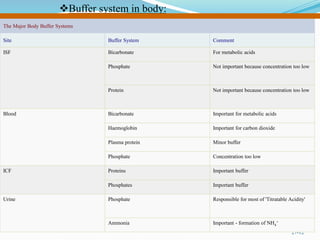
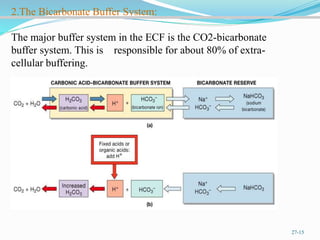
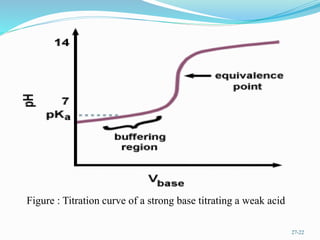
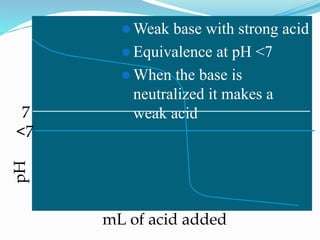
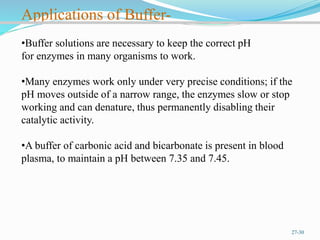
The document provides an overview of buffer solutions, detailing their definition, types (acidic and basic buffers), and the underlying principles that govern their function, such as the Henderson-Hasselbalch equation. It explains the role of different buffer systems in the human body, emphasizing the bicarbonate buffer system and the protein buffer systems in maintaining pH balance. Additionally, it discusses buffer titration and its applications in biological systems, highlighting the importance of buffers in enzyme function and physiological processes.


![27-4
Principles of Buffering:
Isohydric Principle;
All buffer systemswhich participate in defence of
acid - base changes are in equilibrium with each
other. There is after all only one value for [H+] at
any moment. This is known as the Isohydric
Principle.
Buffer solutions achieve their resistance to pH
change because of the presence of an equilibrium
between the acid HA and its conjugate base A-.](https://image.slidesharecdn.com/1-200422181544/85/buffer-by-K-K-Sahu-Sir-4-320.jpg)




![27-9
By definition, pKA = -logKA and pH = -log[H+], so
This equation can then be rearranged to give the Henderson-
Hasselbalch equation](https://image.slidesharecdn.com/1-200422181544/85/buffer-by-K-K-Sahu-Sir-9-320.jpg)