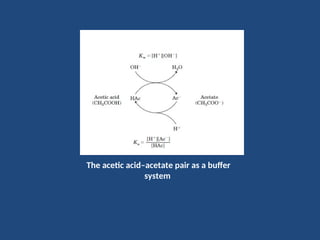
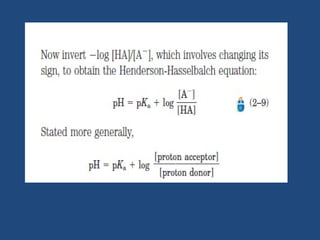
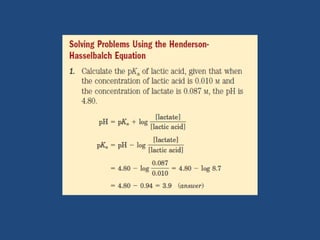
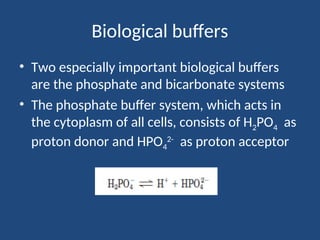
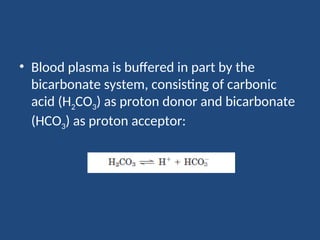
The document discusses the role of buffers in maintaining pH levels in biological systems, emphasizing that even small pH changes can significantly impact biological processes. It defines buffer systems as mixtures of weak acids and their conjugate bases that resist pH changes and introduces the Henderson-Hasselbalch equation for calculating pKa, pH, and molar ratios of proton donors and acceptors. Additionally, it highlights two key biological buffers: the phosphate buffer system in the cytoplasm and the bicarbonate system in blood plasma.