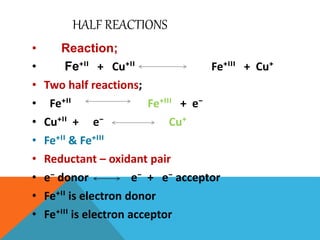
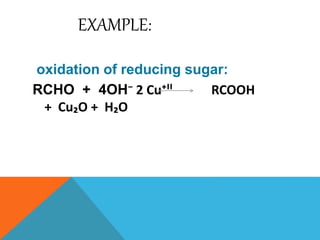
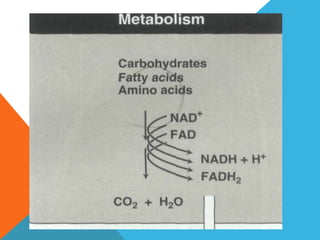
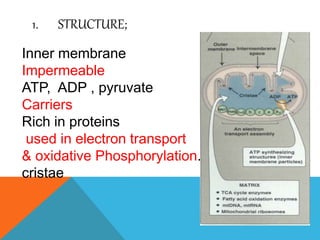
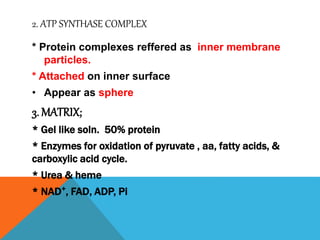
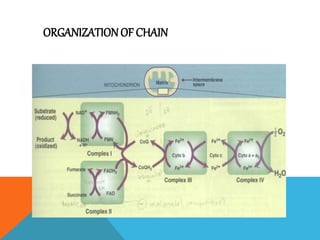
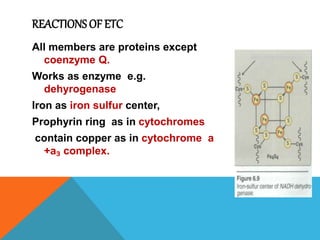
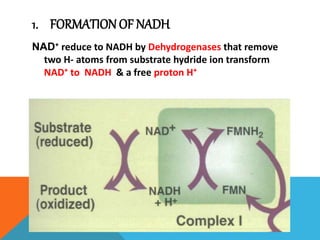
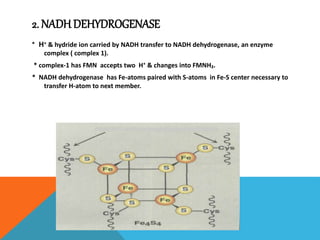
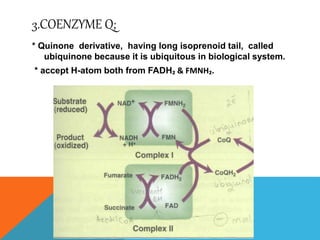
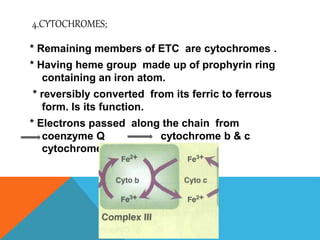
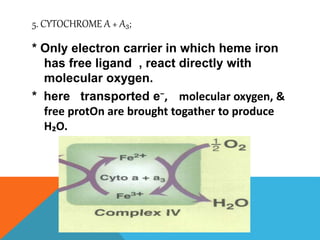
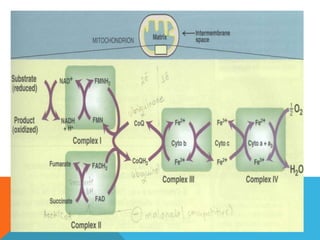
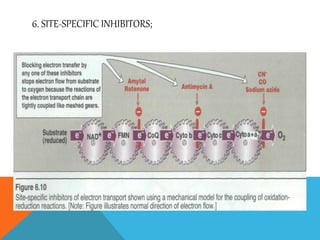
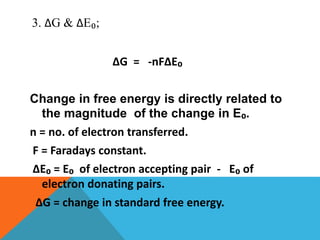
The document discusses biological oxidation and reduction processes, including electron transfer in redox reactions and the role of metabolic pathways. It explains the mechanisms of electron transport chains within mitochondria, detailing the functions of various protein complexes involved in ATP synthesis. Additionally, it covers standard reduction potentials and the relationship between free energy changes and electron transfer.