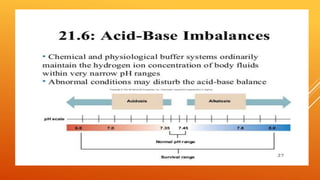
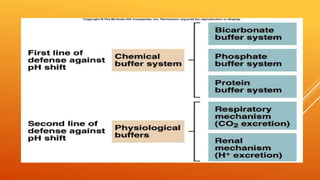
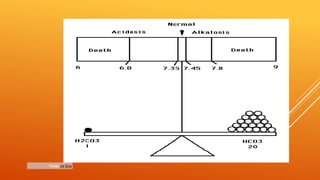
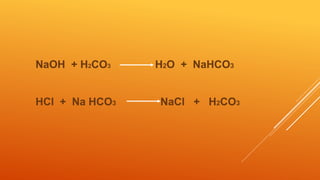
The document discusses two major buffer systems in the body:
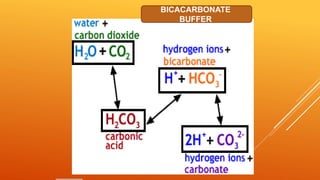
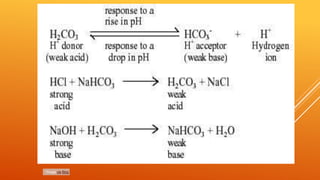
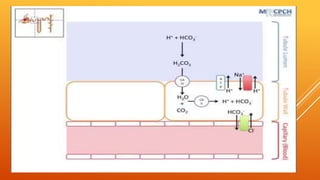
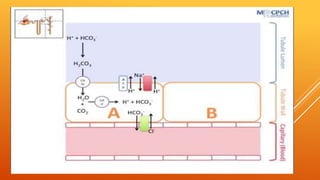
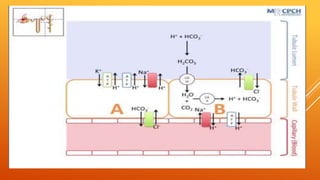
1) The bicarbonate/carbonic acid buffer system is the major plasma buffer. It works to neutralize acids produced from carbon dioxide through the reaction of CO2, H2O and bicarbonate ions. It is regulated by the respiratory and urinary systems.
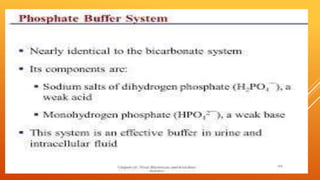
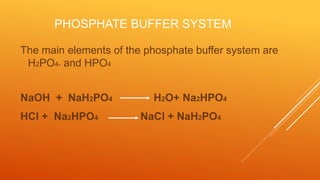
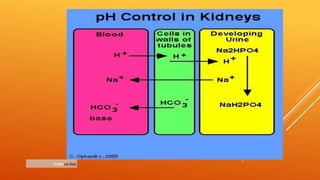
2) The phosphate buffer system is the major buffer in urine and uses hydrogen phosphate and monohydrogen phosphate ions. It has less total buffering power than the bicarbonate system but works to buffer the kidney filtrate and excrete excess acid or base without altering urinary pH.