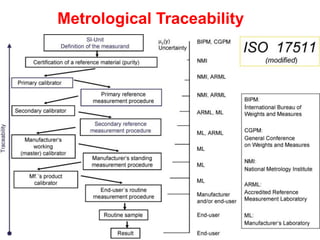
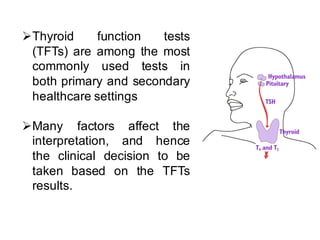
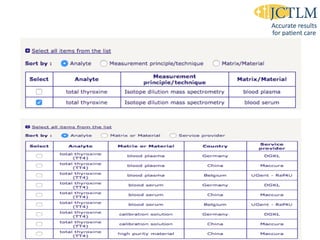
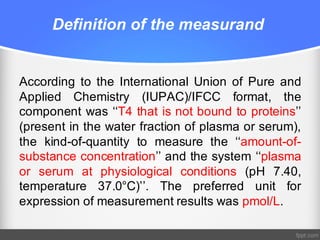
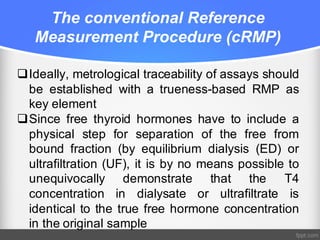
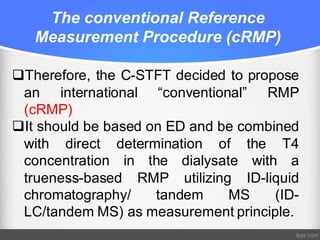
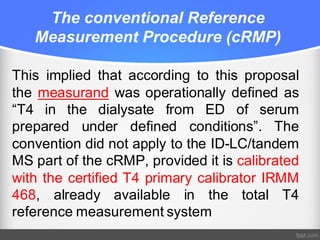
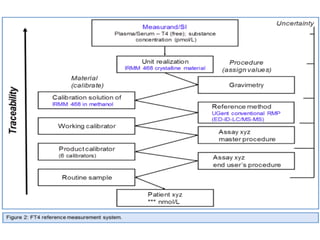
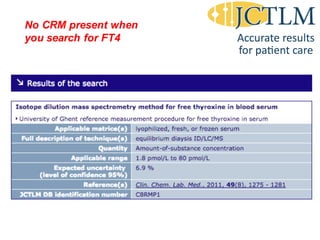
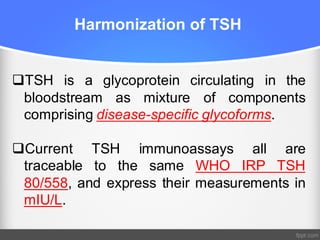
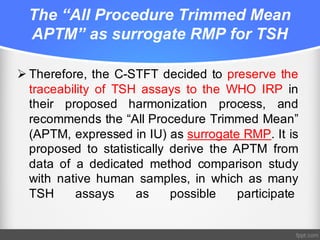
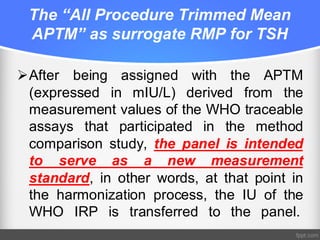
Standardization of thyroid function tests is important to ensure comparable results across laboratories. For tests of total T3 and T4, a reference measurement system exists using primary calibrators and isotope dilution mass spectrometry. For free T3 and T4, a conventional reference measurement procedure was established using equilibrium dialysis and liquid chromatography tandem mass spectrometry. For TSH, the measurand was defined as intact total TSH accounting for glycosylation, and an "All Procedure Trimmed Mean" statistical approach using multiple commercial assays on patient samples was adopted as a surrogate reference measurement procedure.




![UK NEQAS [Edinburgh], Department ofLaboratoryMedicine,Royal Infirmary ofEdinburgh,Edinburgh EH164SA,UK](https://image.slidesharecdn.com/standardizationofthyroidfunctiontestsolaelgaddar05-05-2017-170310110054/85/Standardization-of-Thyroid-Function-Tests-05-05-2017-5-320.jpg)