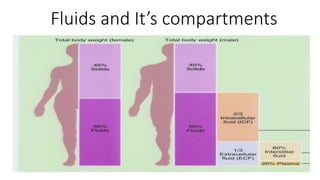
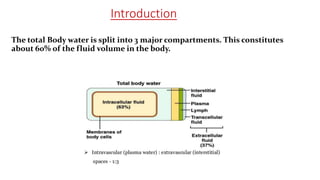
The document discusses fluid and electrolyte balance in the human body. It describes the three major fluid compartments - plasma, interstitial fluid, and intracellular fluid. Plasma and interstitial fluid make up the extracellular fluid compartment, while intracellular fluid is contained within cells. Key points include:
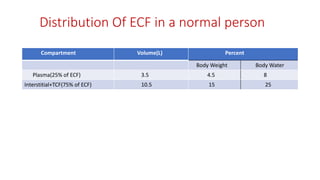
- Extracellular fluid is around 25% of total body water and includes plasma (3.5L), interstitial fluid (10.5L), and transcellular fluid.
- Intracellular fluid contains around 28L and maintains osmotic equilibrium with the extracellular compartment.
- Important ions like sodium, potassium, calcium, and chloride are regulated and maintained within tight concentration ranges between fluid compartments



![• The intracellular fluid, also known as cytosol, is all
fluid contained inside the cells.[3] It is the matrix in
which cellular organelles are suspended.
• The cytosol and organelles together compose
the cytoplasm.
• The cell membranes are the outer barrier. In
humans, the intracellular compartment contains on
average about 28 litres of fluid, and under ordinary
circumstances remains in osmotic equilibrium.
• It contains moderate quantities of magnesium and
sulphate ions.
• In the cell nucleus the fluid component of
the nucleoplasm is called the nucleosol.](https://image.slidesharecdn.com/fluidandelectrolytebalancefinal-240205162604-24bd4727/85/Fluid-And-electrolyte-Balance-FINAL-pptx-8-320.jpg)

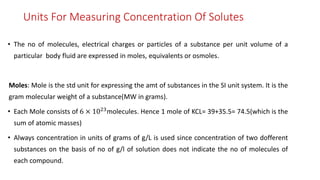



![• Osmotic concentration, formerly known as osmolarity is the measure
of solute concentration, defined as the number of osmoles (Osm) of solute per litre (L)
of solution (osmol/L or Osm/L).
• The osmolarity of a solution is usually expressed as Osm/L (pronounced "osmolar"), ie
mole of solute particles.
• Whereas molarity measures the number of moles of solute per unit volume of solution,
measures the number of osmoles of solute particles per unit volume of solution.[2]
• allows the measurement of the osmotic pressure of a solution and the determination
solvent will diffuse across a semipermeable membrane (osmosis) separating two
different osmotic concentration.](https://image.slidesharecdn.com/fluidandelectrolytebalancefinal-240205162604-24bd4727/85/Fluid-And-electrolyte-Balance-FINAL-pptx-15-320.jpg)

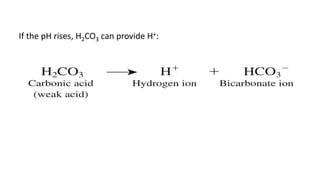
![Concept Of pH And H+ Concentration:
H+ concentration of various body fluids is expressed in two different ways, either directly(H+) or
indirectly as pH.
pH refers to –ve logarithm of the H+. The relation b/w H+ & pH can be expressed.
i. pH= log10 1/[H+]
ii. pH= -log[H+]
2. pH and H+ are inversely related: When the pk of a buffer system is known the effective pH range
of buffer is known.
Here in pK….K= Ionisation constant. ∴ pK= -logK and is equal to the pH at which half of acid
molecules are dissociated and half are undissociated.](https://image.slidesharecdn.com/fluidandelectrolytebalancefinal-240205162604-24bd4727/85/Fluid-And-electrolyte-Balance-FINAL-pptx-17-320.jpg)

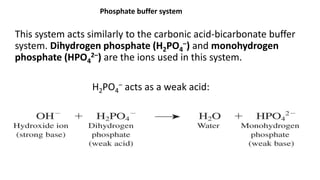
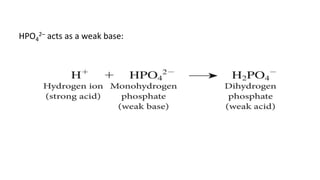
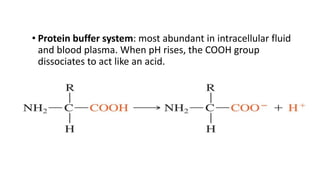
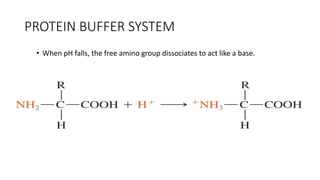
![Dynamics Of Equilibrium
• The general equation for buffer system: Henderson-Hasselbalch Equation
pH: Acidty of a buffer solution
pKa: negative log of Ka(ionisation constant)
Ka: Acid dissociation constant
[A-]: concn of acid
[HA]: concn of conjugate base
• can be used to estimate the pH of a buffer solution. The numerical
value of the acid dissociation constant, Ka, of the acid is known or
assumed.
• The equilibrium between the weak acid and its conjugate base
allows the solution to resist changes to pH when small amounts of
strong acid or base are added.](https://image.slidesharecdn.com/fluidandelectrolytebalancefinal-240205162604-24bd4727/85/Fluid-And-electrolyte-Balance-FINAL-pptx-26-320.jpg)
![• The Henderson–Hasselbalch equation relates the pH of a solution
containing a mixture of the two components to the acid
dissociation constant, Ka, and the concentrations of the species in
solution.[3] To derive the equation a number of simplifying
assumptions have to be made.](https://image.slidesharecdn.com/fluidandelectrolytebalancefinal-240205162604-24bd4727/85/Fluid-And-electrolyte-Balance-FINAL-pptx-27-320.jpg)
