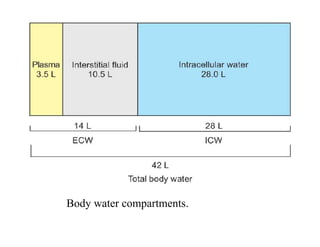
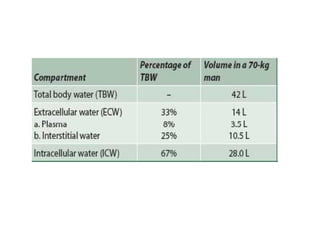
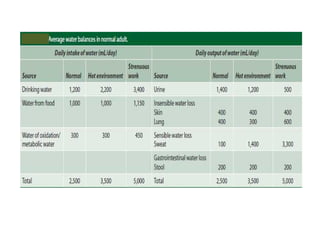
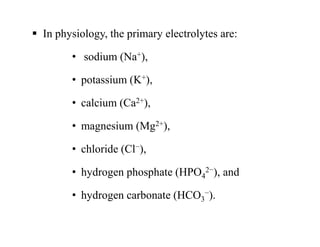
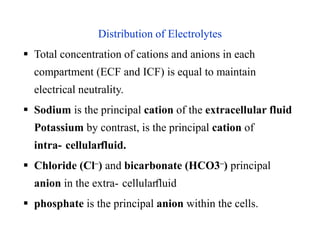
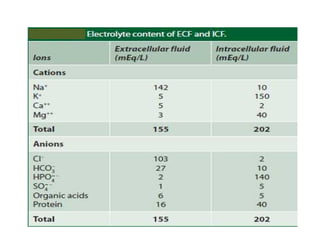
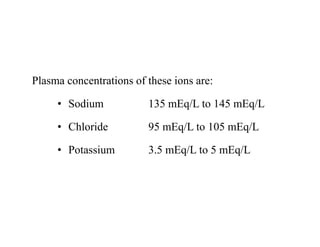
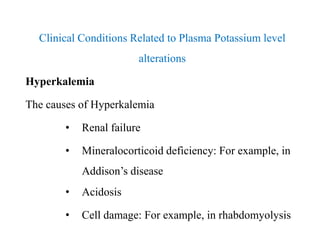
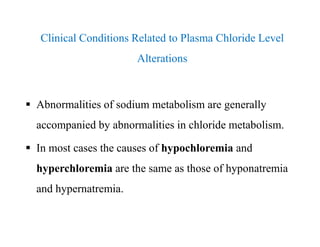
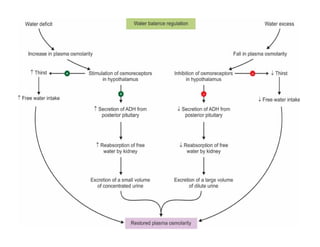
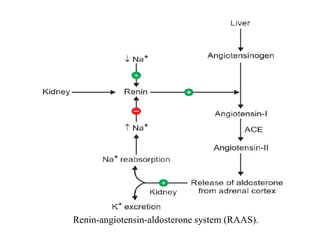
The document discusses water and electrolytes in the human body. It states that water makes up 60-70% of body mass and is highest in newborns at 75% and decreases with age to less than 50% in older individuals. Water is involved in metabolic reactions, transport of solutes, regulation of temperature, lubrication of joints, and maintenance of cell structure. Total body water is about 42 liters in a 70kg adult, divided into intracellular and extracellular compartments. Electrolytes like sodium, potassium, chloride, and bicarbonate are important ions that are regulated between intracellular and extracellular fluids.