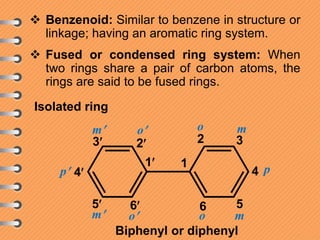
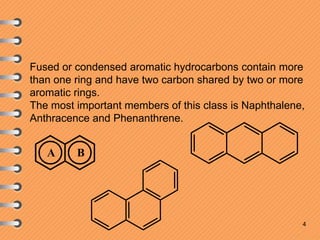
The document discusses polynuclear aromatic hydrocarbons, primarily focusing on naphthalene, anthracene, and phenanthrene. It covers their definitions, structures, physical and chemical properties, as well as methods of synthesis and reactivity, highlighting the electrophilic substitution reactions and resonance energy differences among these compounds. Key aspects include the stability of their structures and the resulting isomers from substitution reactions.





















![[HNO3+H2SO4 is not used, leads formation
of 9,10 anthraqunone by oxidation]
Reduction
Friedel-crafts acylation](https://image.slidesharecdn.com/fusedpolynuclearcompounds-200806050638/85/Fused-polynuclear-compounds-24-320.jpg)













