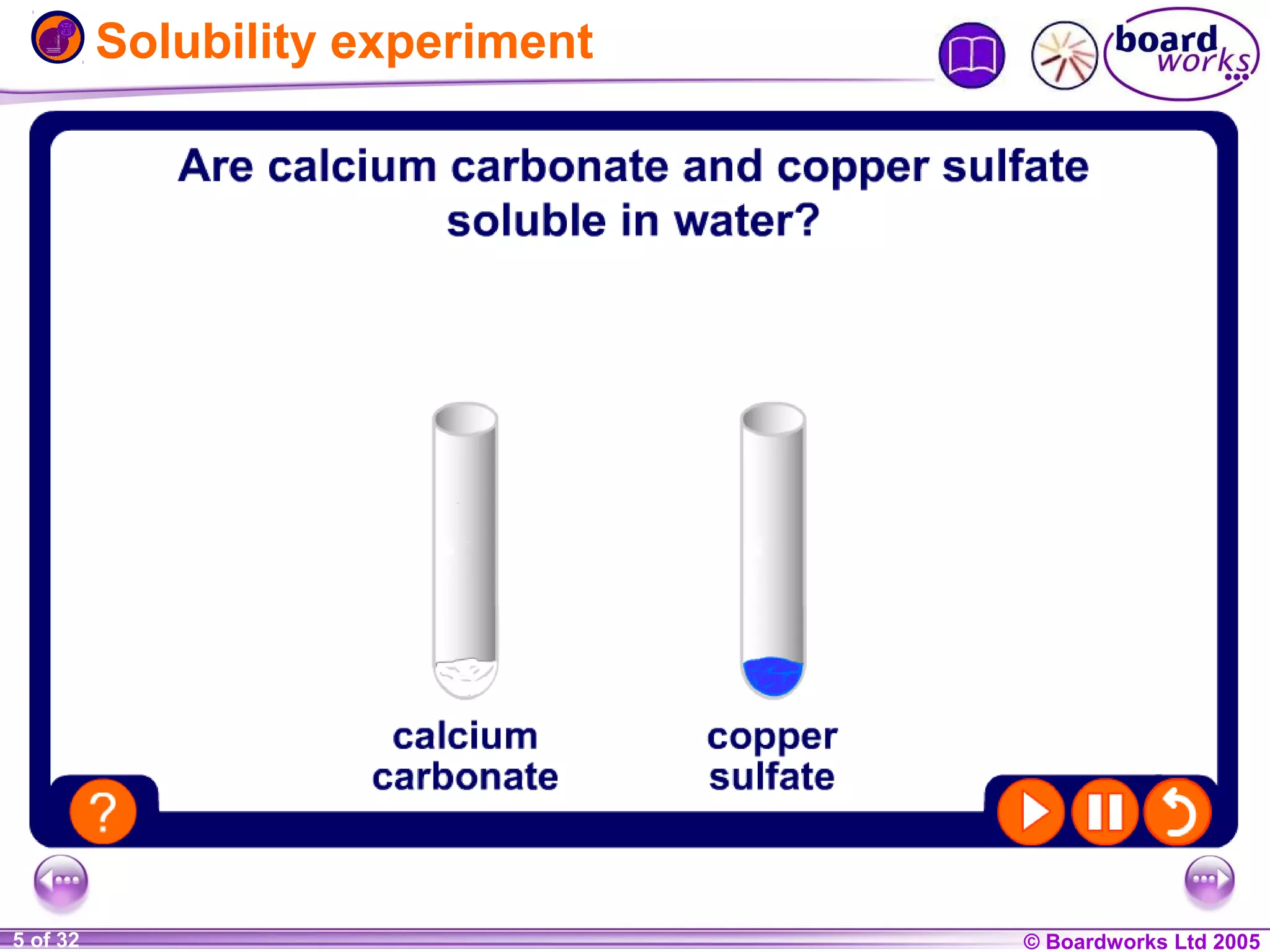
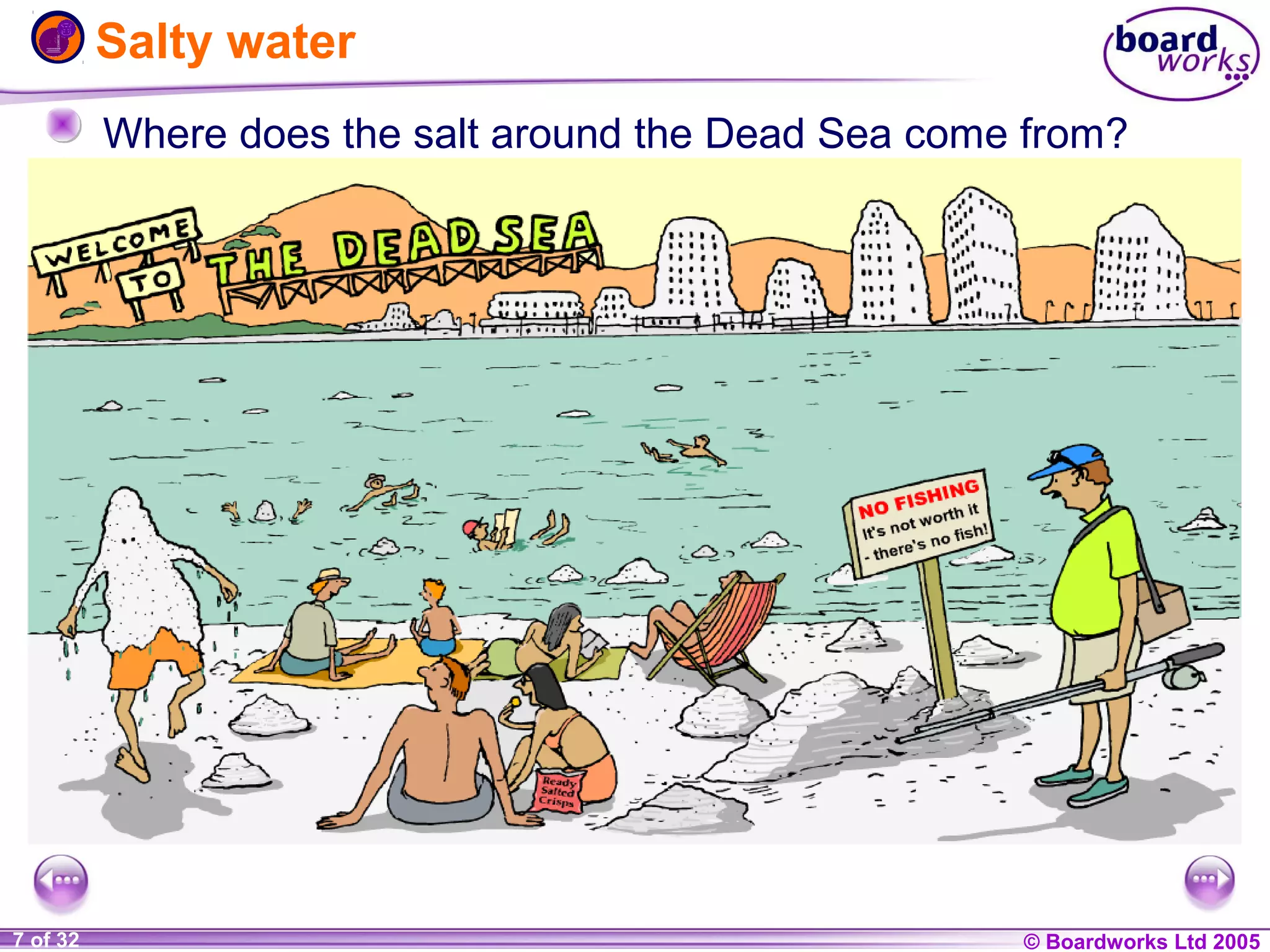
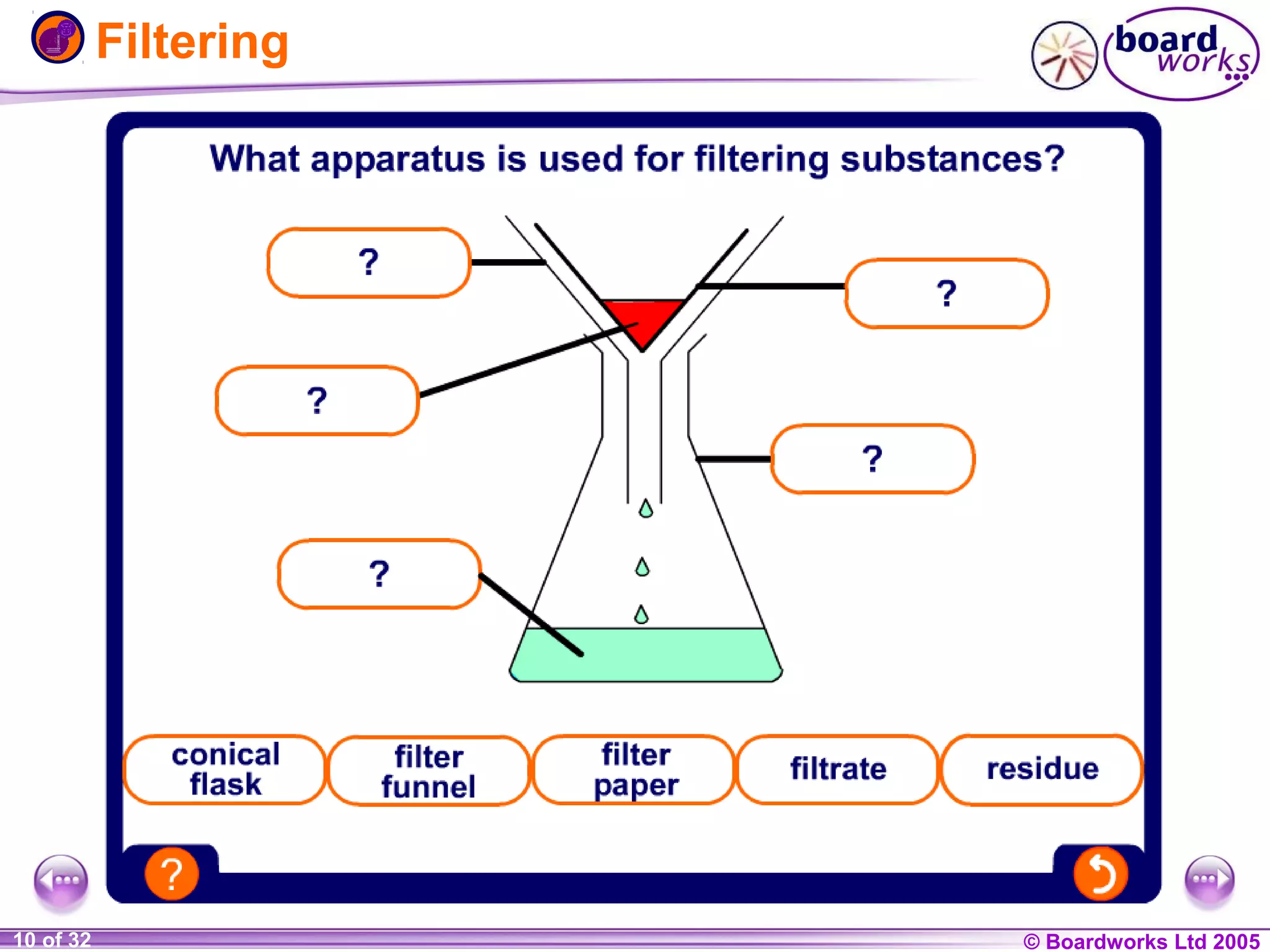
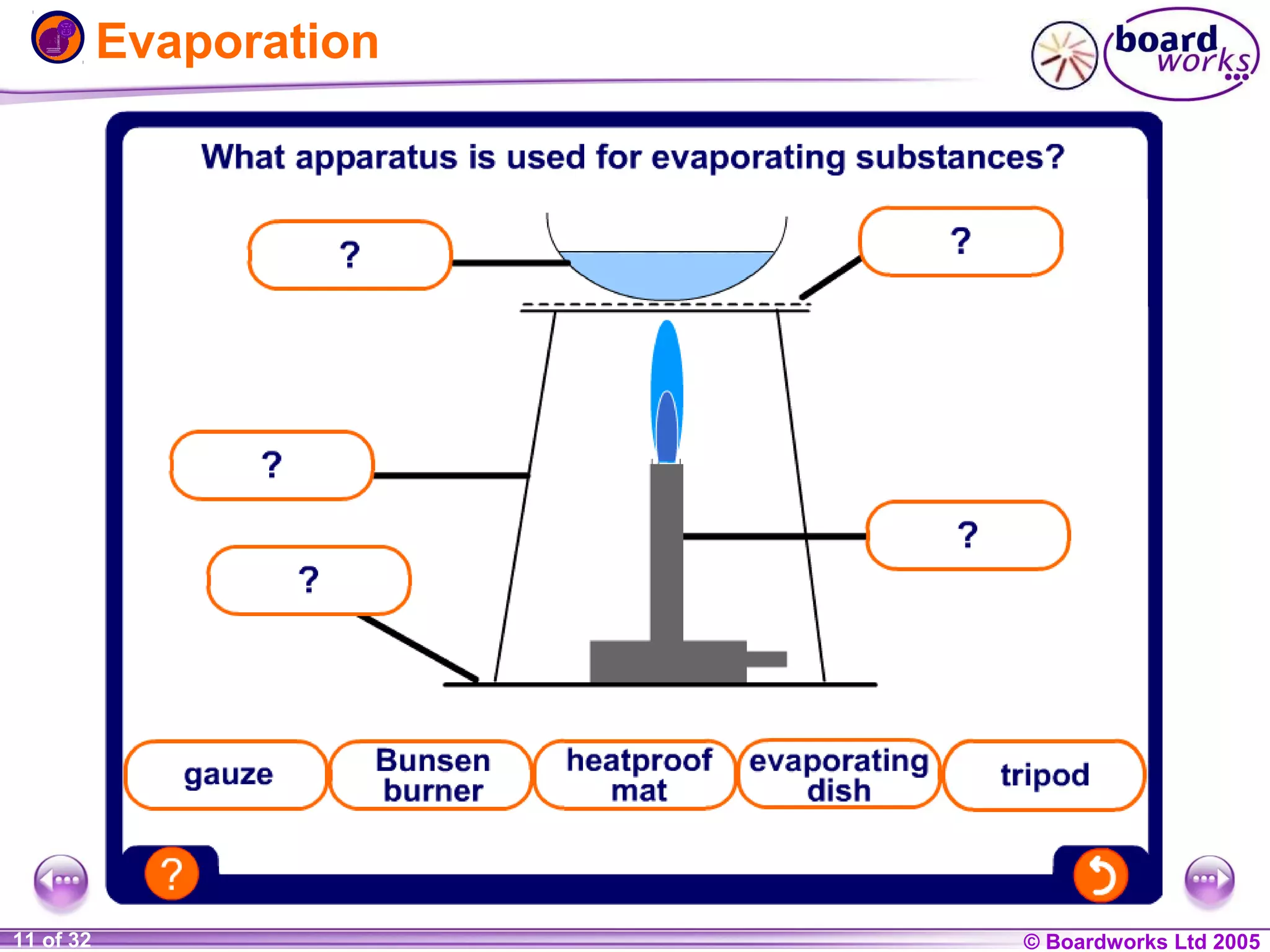
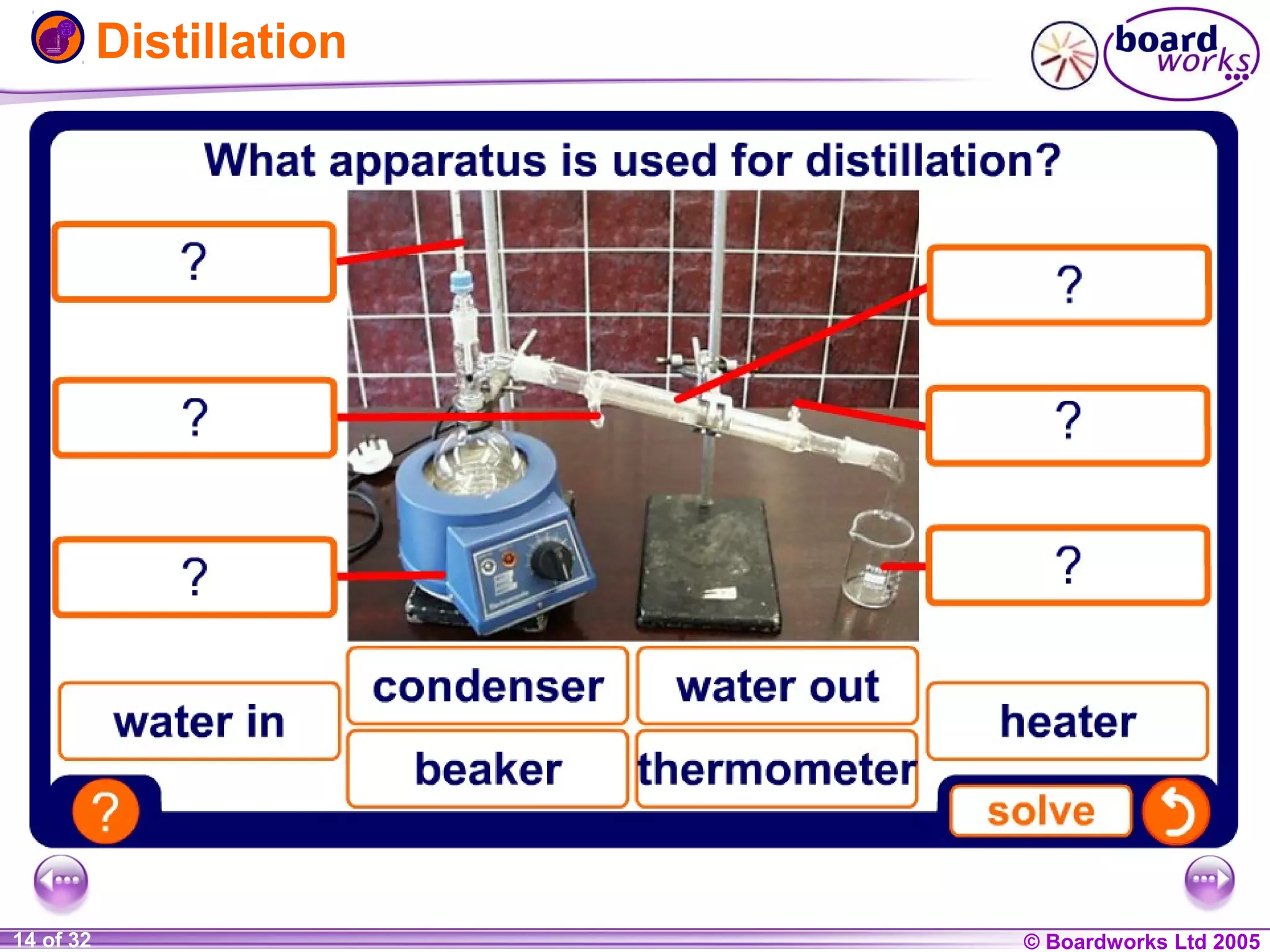
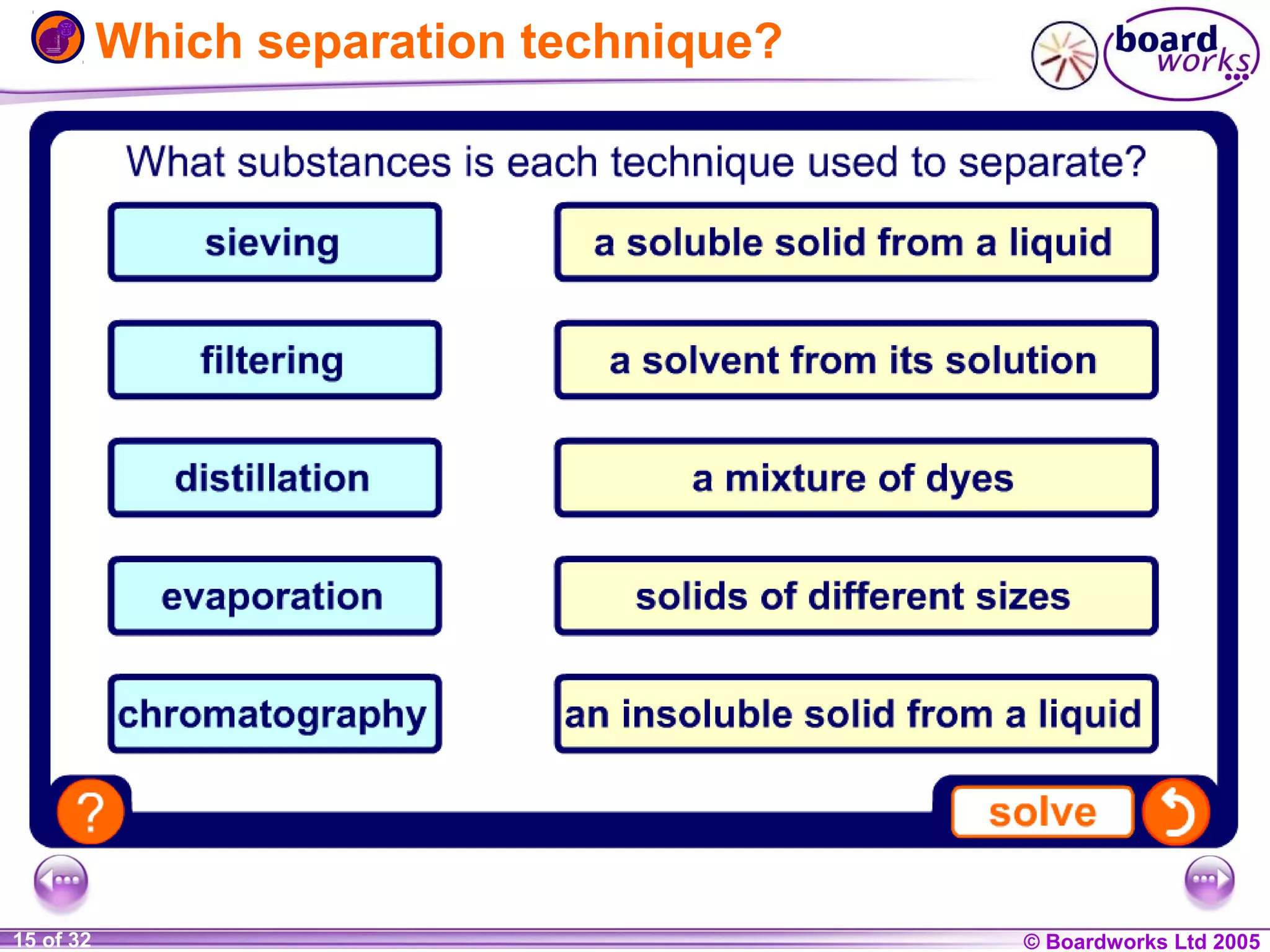
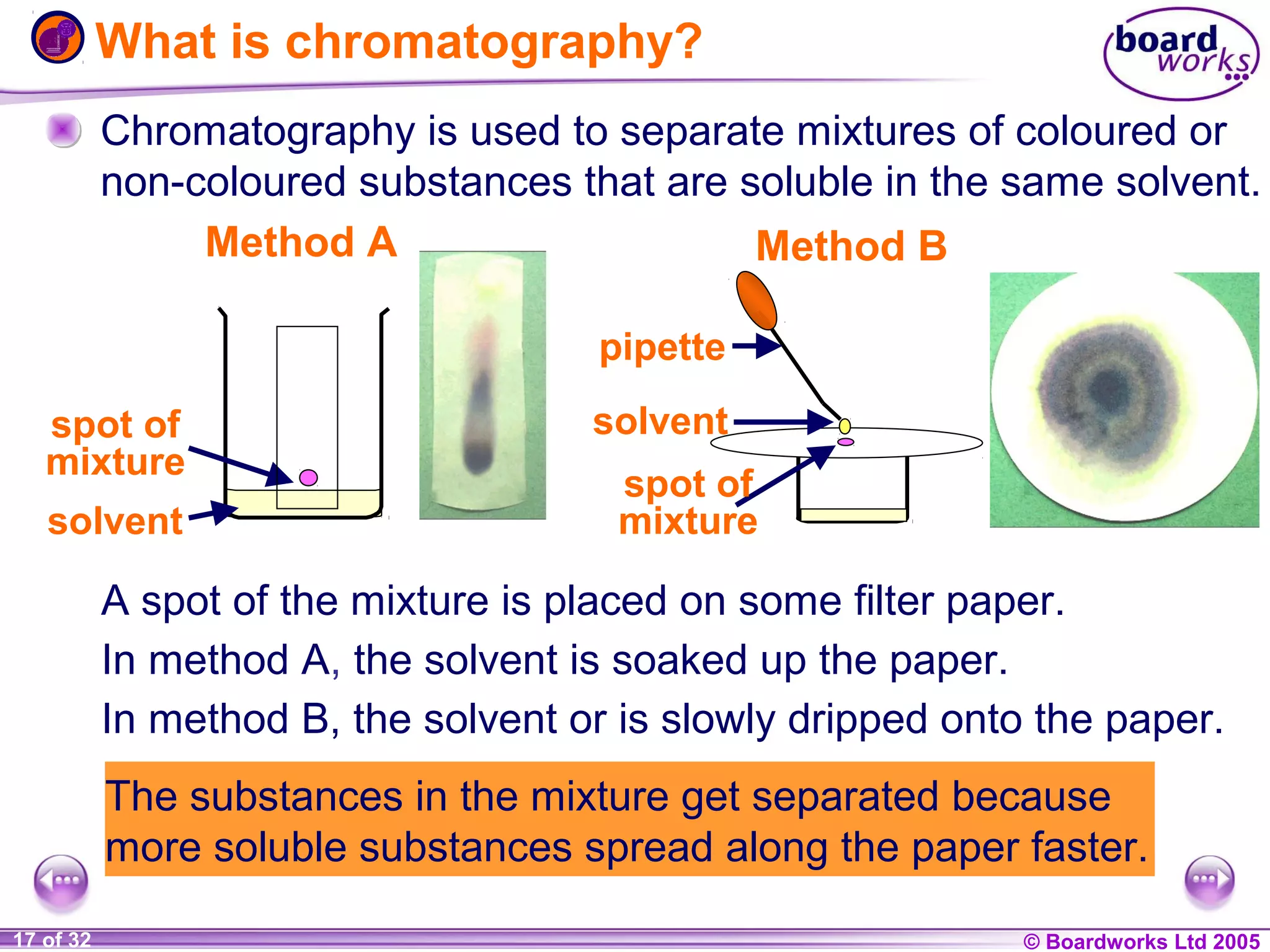
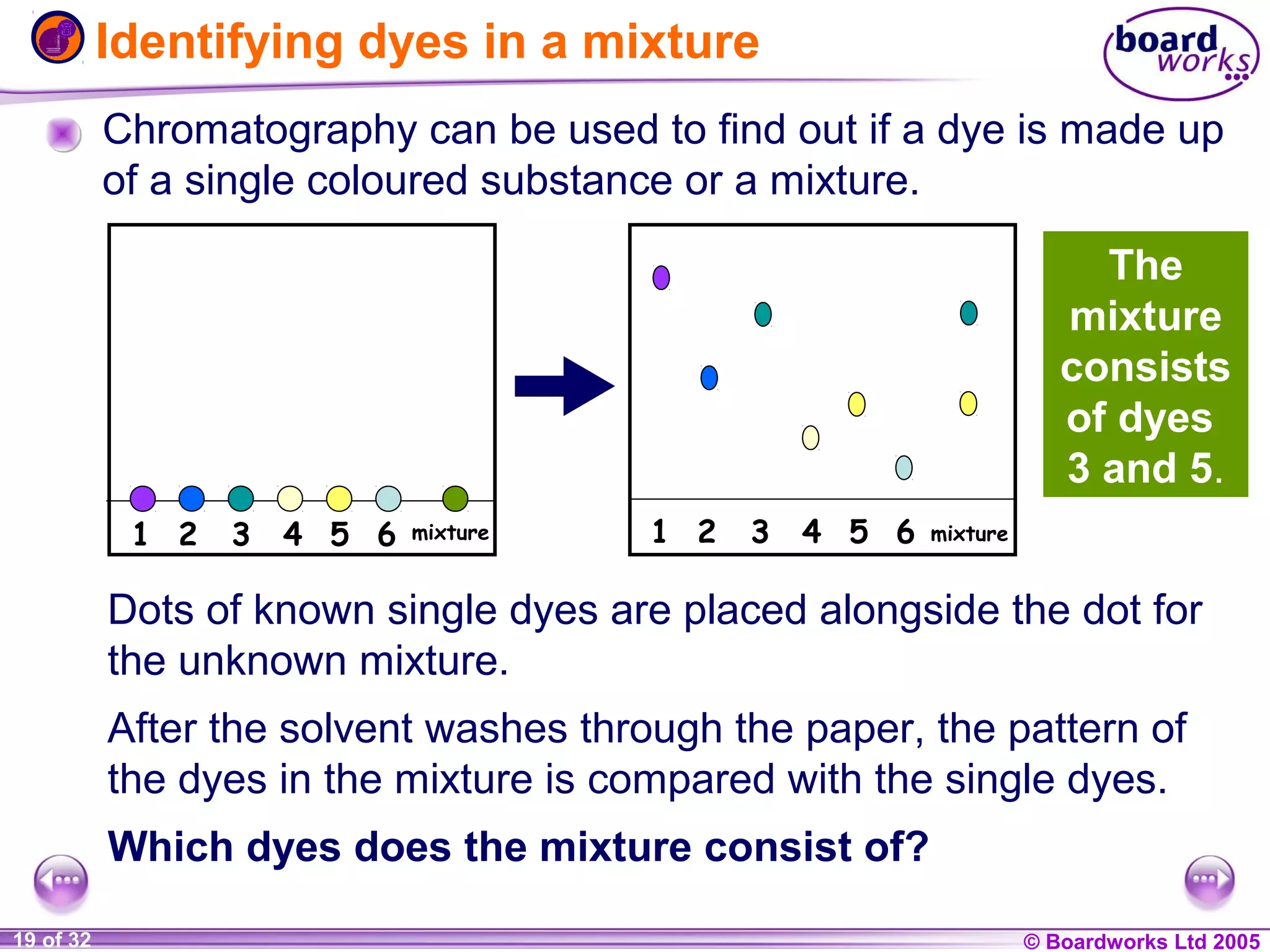
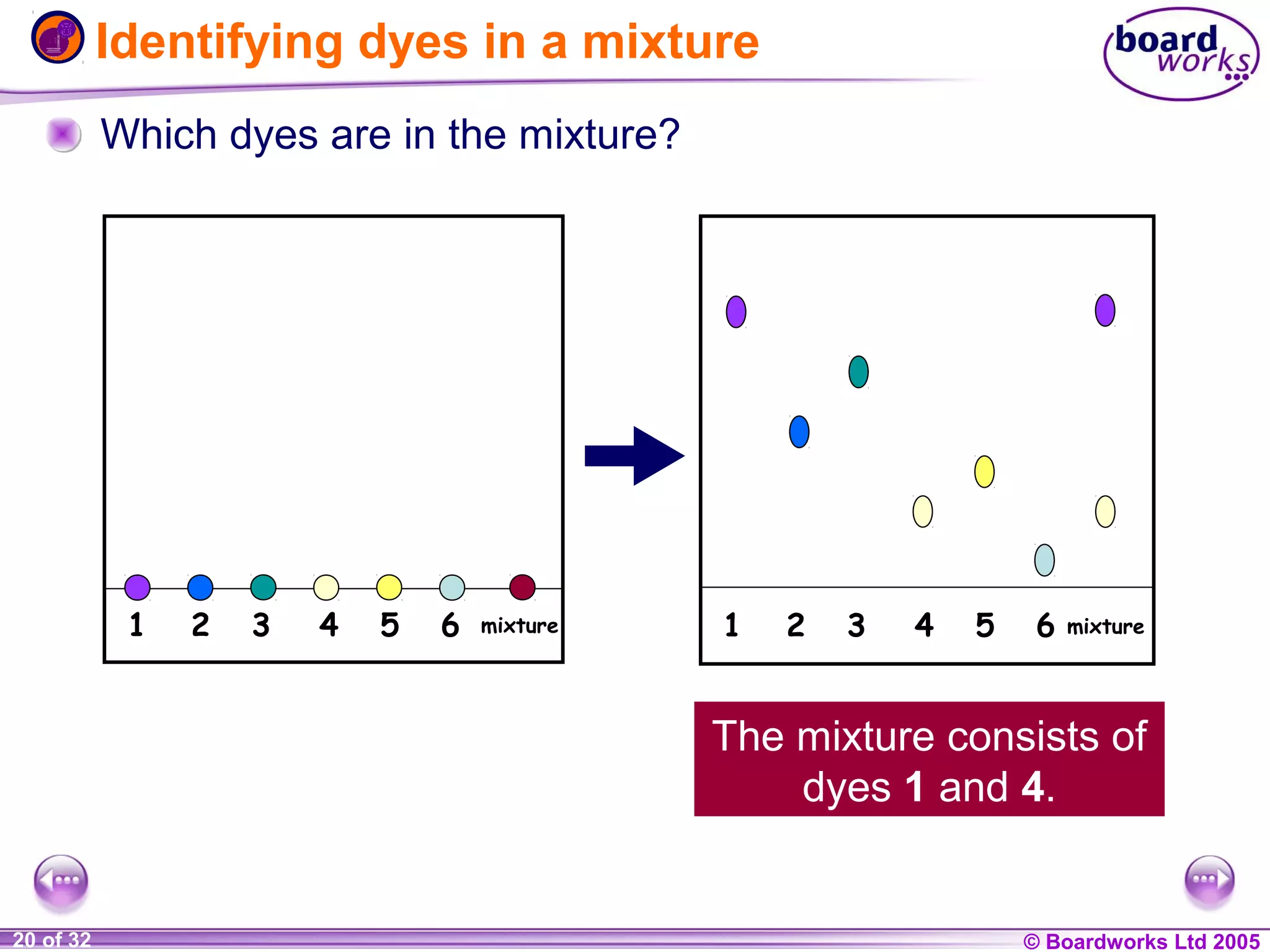
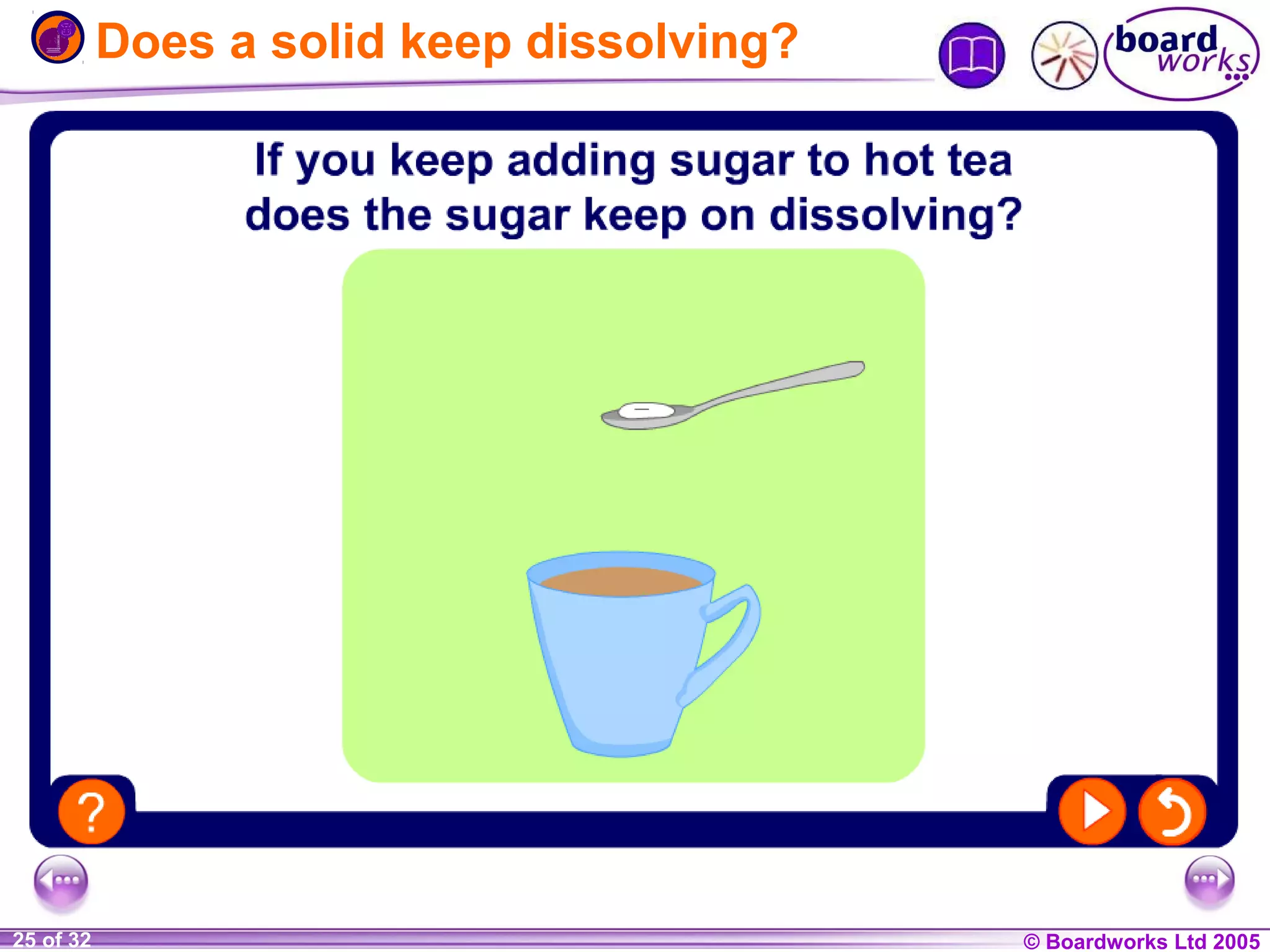
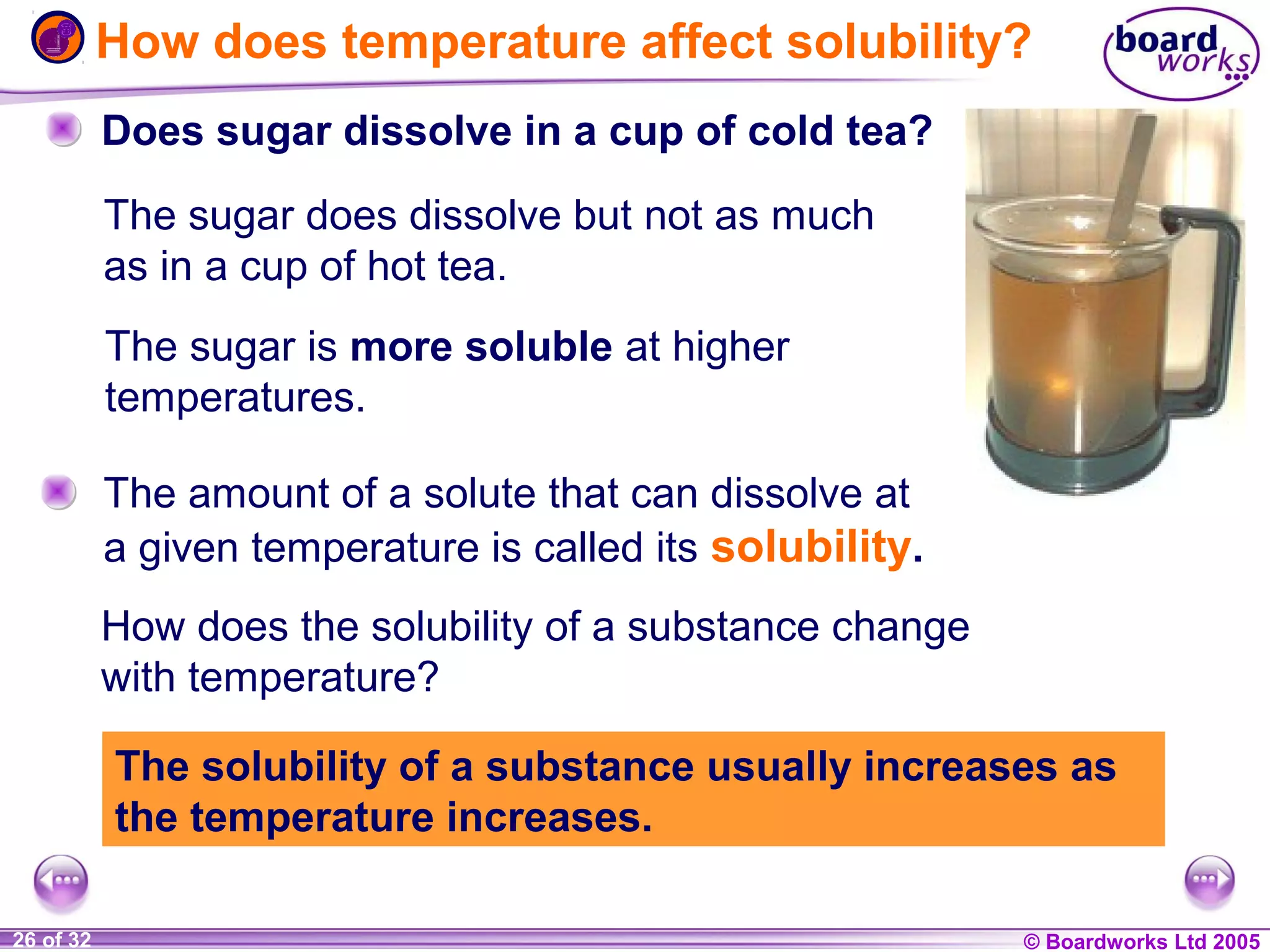
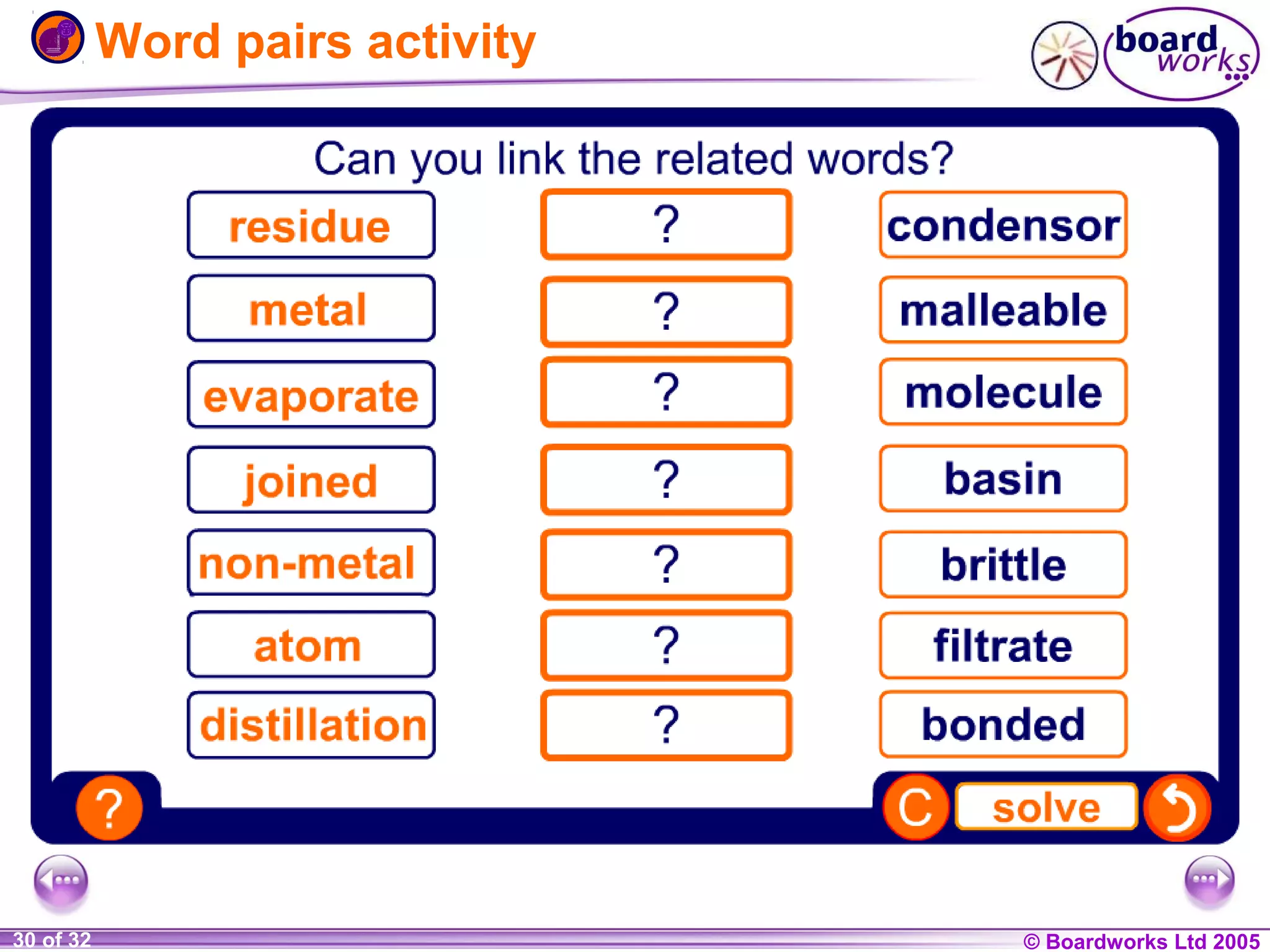
The document is a chemistry textbook section about solutions. It introduces key concepts like mixtures, solutions, solutes and solvents. It describes different techniques for separating mixtures, including filtration, evaporation, distillation and chromatography. It also discusses how solubility is affected by temperature and saturation.