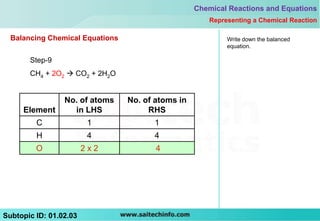
The document outlines the 9 step process for balancing a chemical equation:
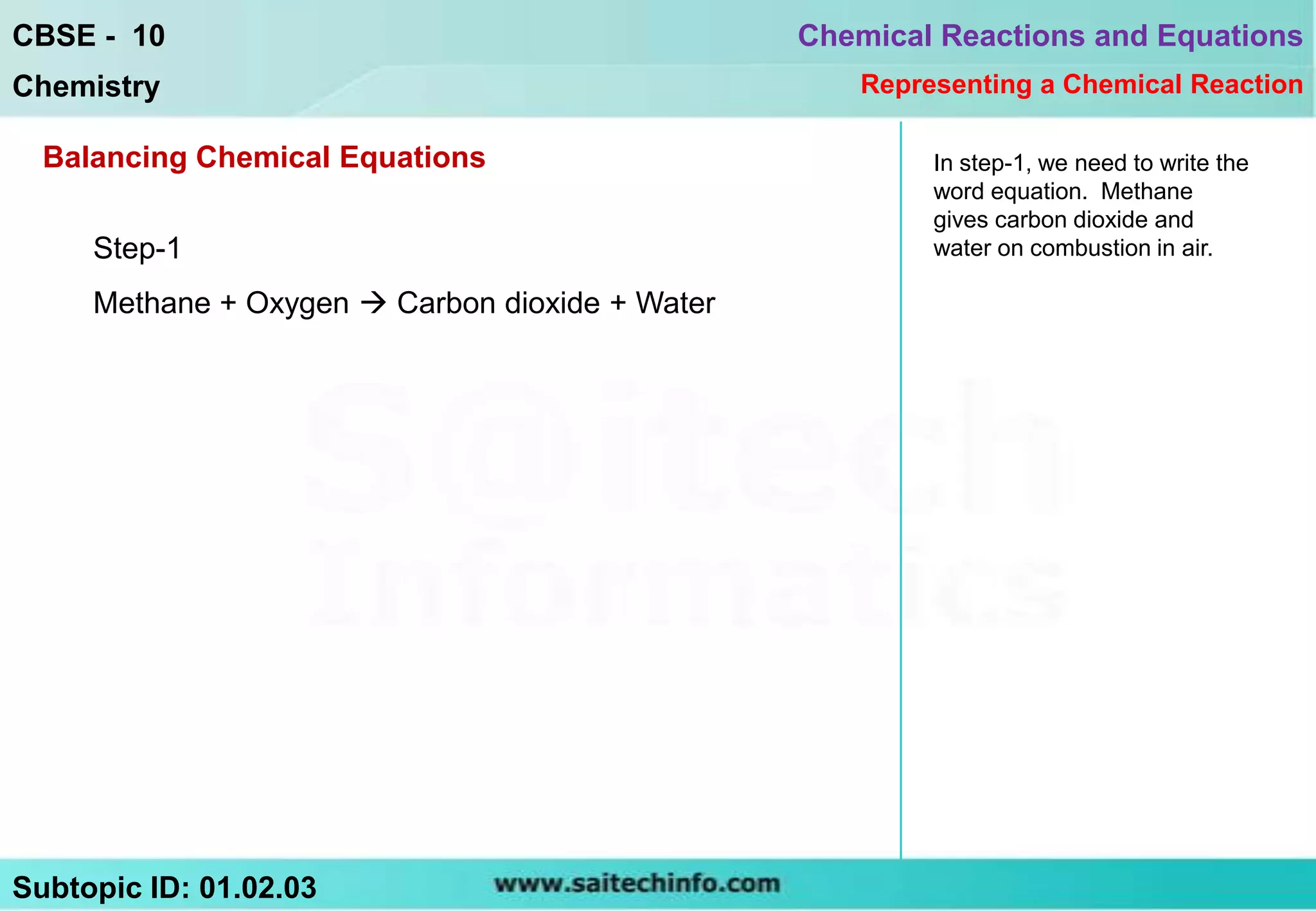
1) Write the word equation
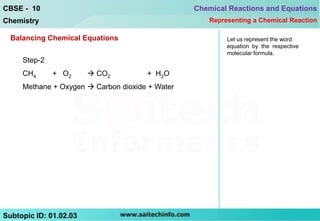
2) Convert to molecular formula
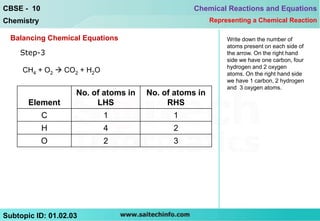
3) Write the number of atoms on each side
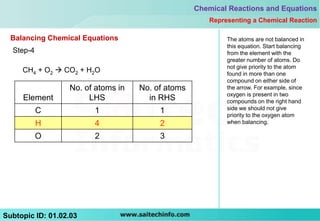
4) Balance the side with more atoms of each element
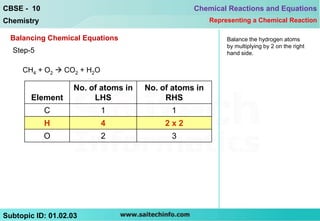
5) Balance hydrogen atoms first if needed
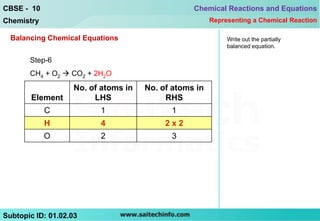
6) Write the partially balanced equation
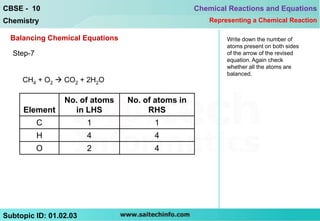
7) Check atom counts again
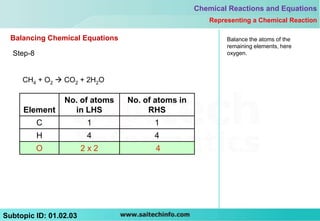
8) Balance the remaining elements
9) Write the fully balanced chemical equation