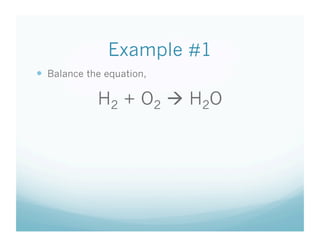
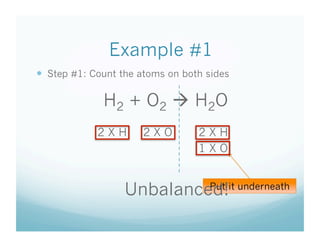
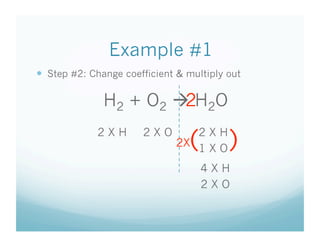
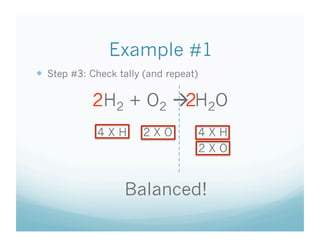
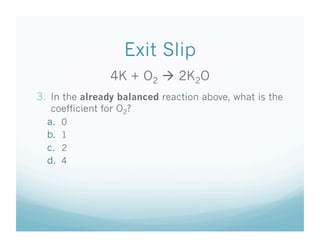
The document provides an overview of a chemistry lesson on balancing chemical equations, including examples of balancing the equations H2 + O2 → H2O and H2 + Cl2 → HCl. It outlines the 3-step process for balancing equations: counting atoms on both sides, changing coefficients and multiplying, and checking the tally. Practice questions are assigned as homework.