Embed presentation
Downloaded 127 times


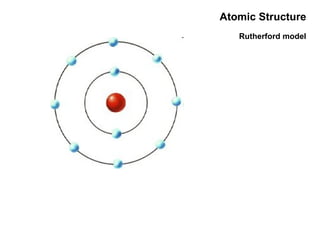
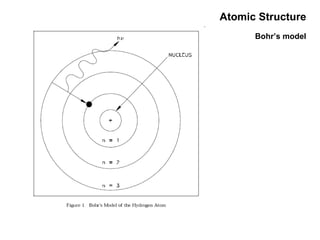
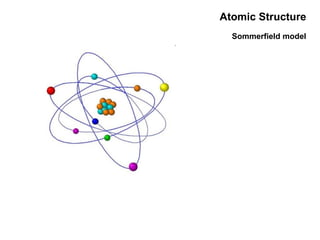
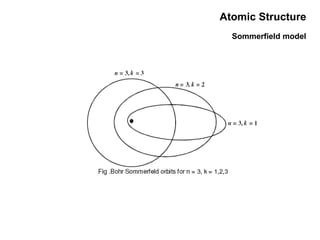
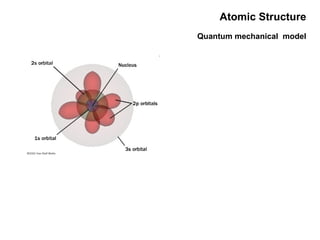
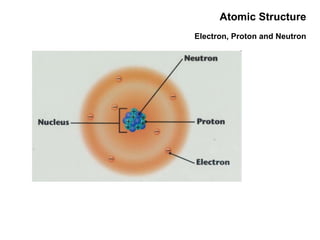
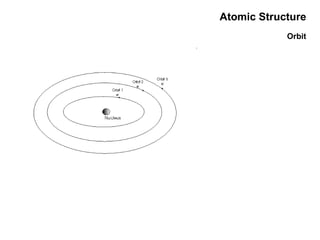
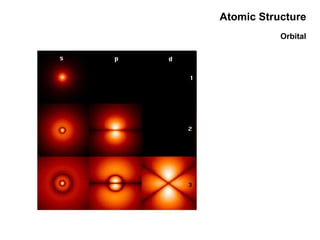
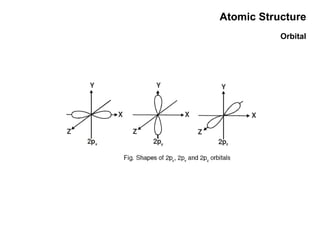
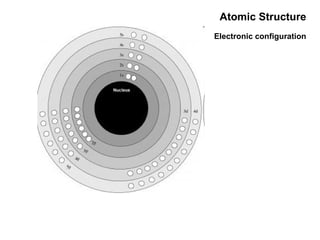
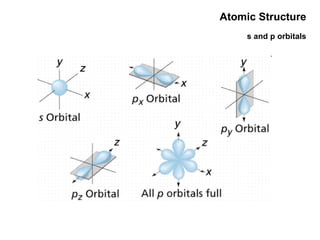
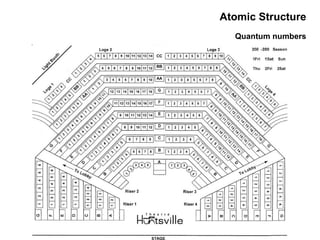
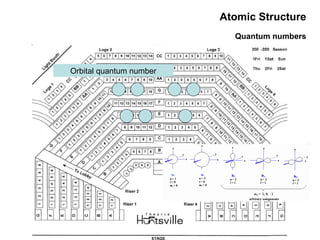
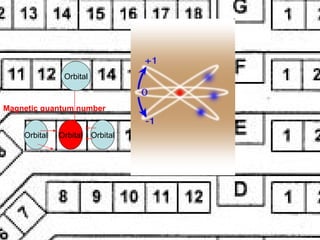
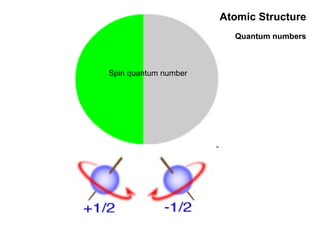
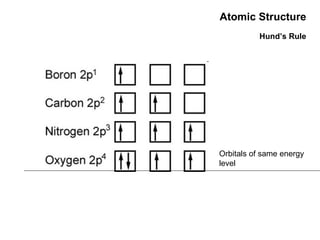
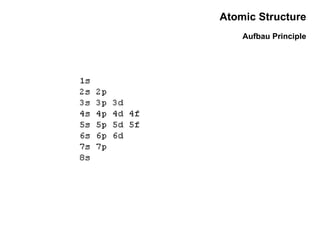
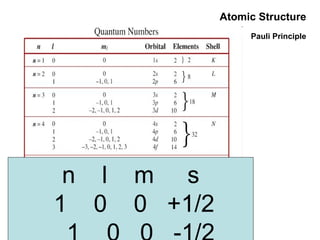


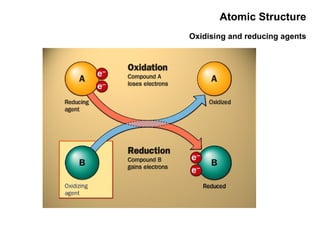

This document discusses the evolution of atomic structure models from Dalton's model to the current quantum mechanical model. It introduces key atomic concepts such as electrons, protons, neutrons, orbitals, quantum numbers including principal, orbital, magnetic and spin quantum numbers. It also covers electronic configuration, Hund's rule, the Aufbau principle, Pauli principle, and oxidation-reduction concepts including oxidation states and oxidizing and reducing agents.
























