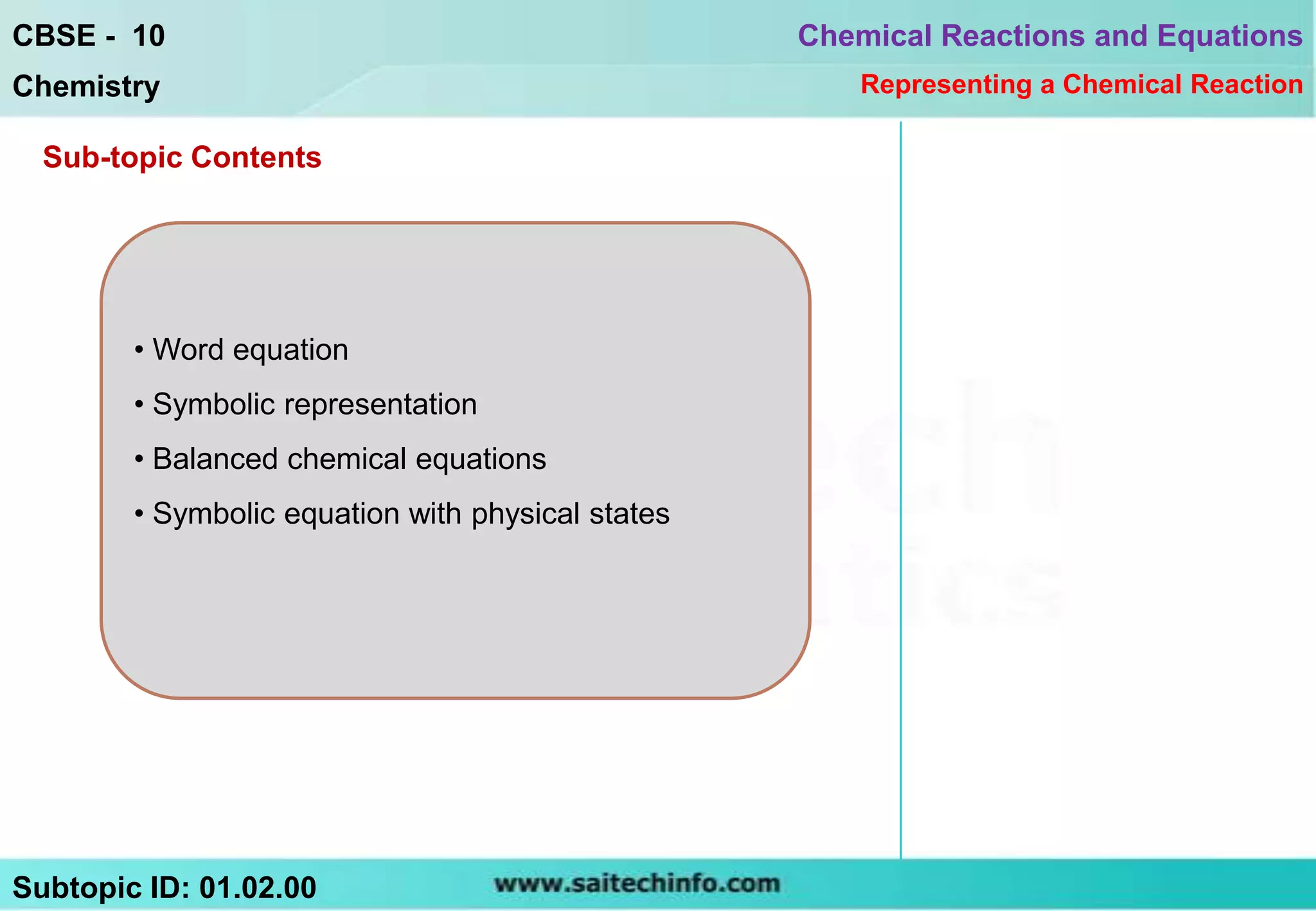
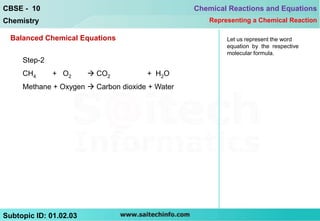
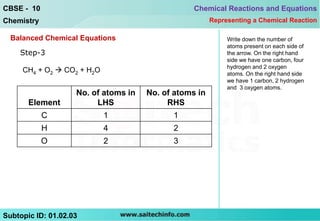
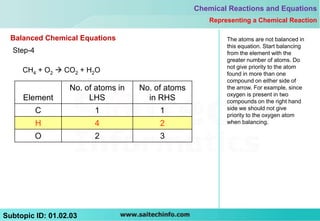
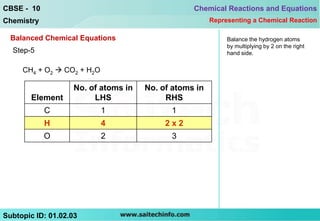
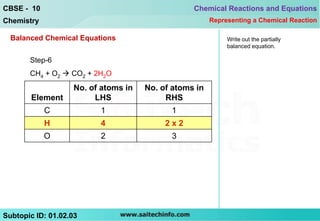
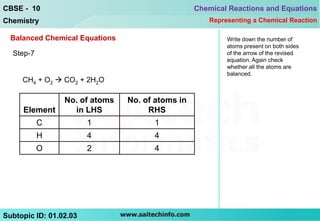
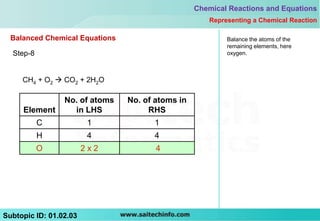
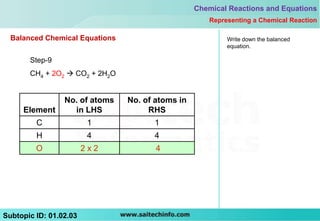
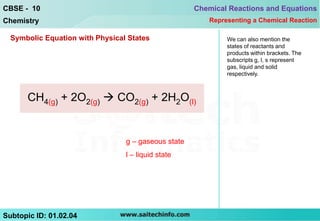
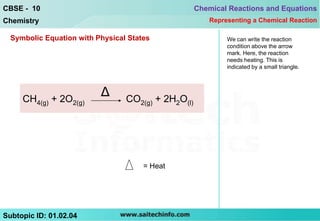
This document discusses different ways to represent a chemical reaction, including word equations, symbolic representations, and balanced chemical equations. It provides a step-by-step example of balancing the chemical equation for the combustion of methane. The key steps are writing the word equation, representing it with molecular formulas, determining the atoms on each side, and adjusting coefficients as needed to balance each atom. A balanced chemical equation can also specify the physical states of reactants and products using subscripts and indicate reaction conditions above the arrow.