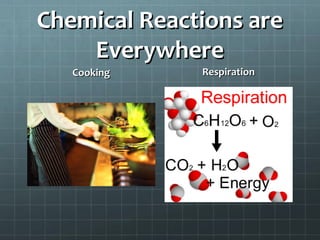
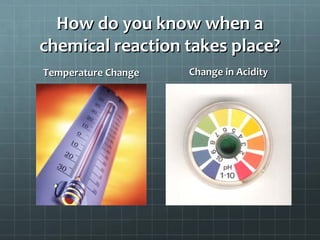
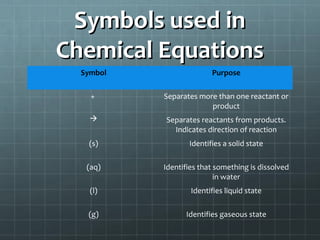
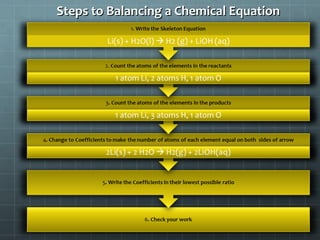
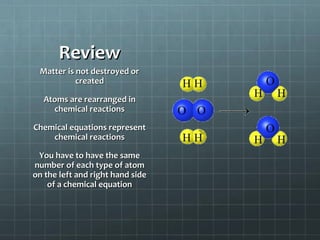
This document provides an introduction to chemical reactions and how they are represented by chemical equations. It explains that chemical equations are balanced to show the same number of atoms of each element on both sides, as required by the law of conservation of mass which states that atoms are neither created nor destroyed in a chemical reaction. It also gives examples of how to identify chemical reactions through observations of color change, gas formation, temperature change and more. Finally, it demonstrates how to balance chemical equations by ensuring the same number of each type of atom is on both sides of the equation.