Embed presentation

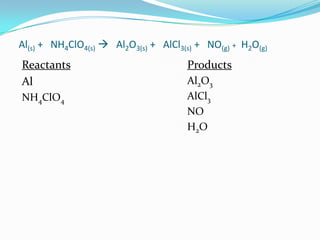
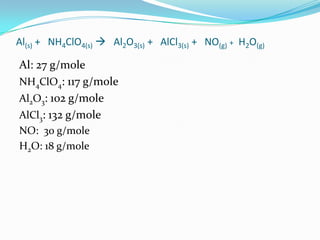






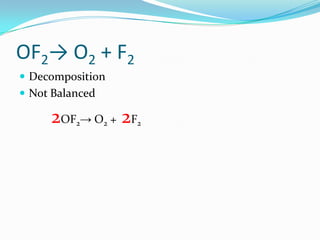






The document provides an example chemical reaction and asks the student to: 1) Identify the reactants and products and write them in separate columns 2) Balance the chemical equation 3) Calculate the molar mass of each compound It then gives the balanced equation, reactants, products, and molar masses. The next section provides examples of unbalanced equations for students to balance, covering different reaction types like decomposition, single replacement, double replacement, combustion, and more. It concludes with a definition of an atom inventory as counting the atoms in a compound.














