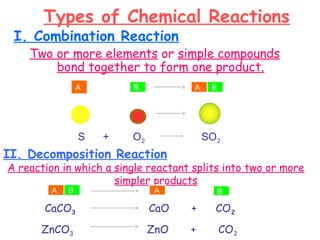
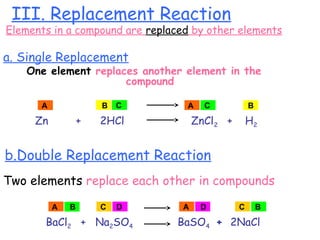
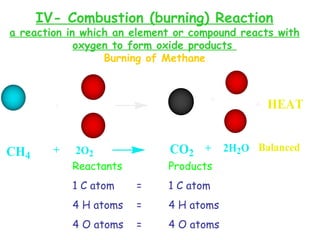
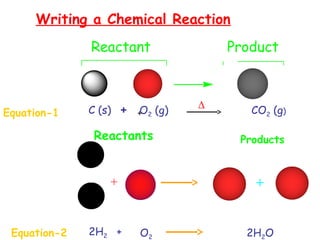
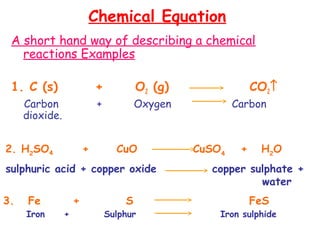
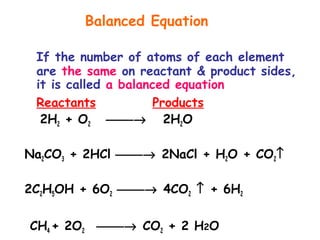
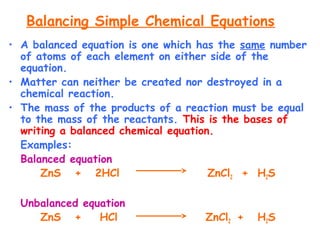
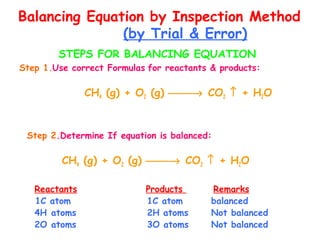
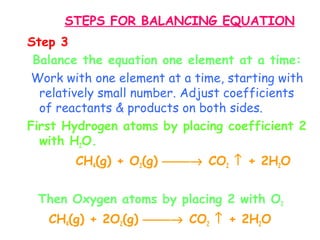
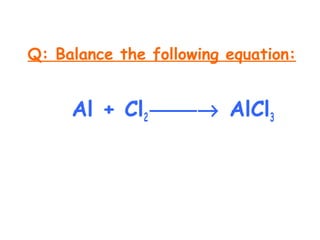
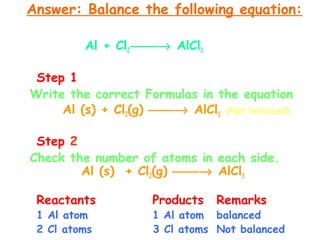
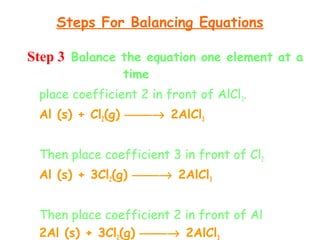
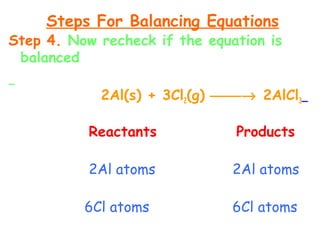
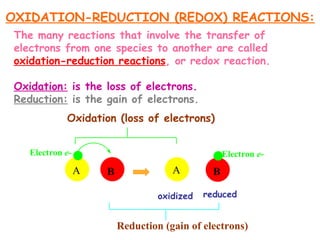
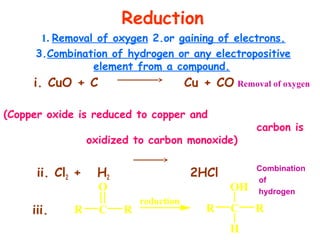
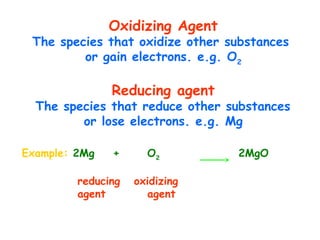
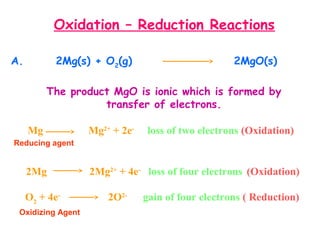
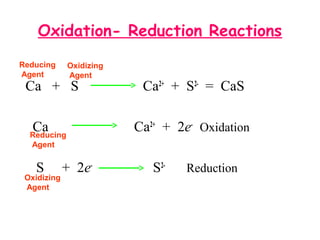
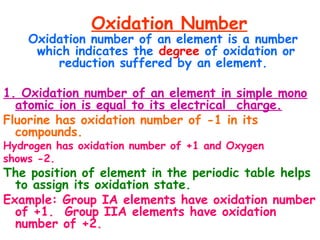
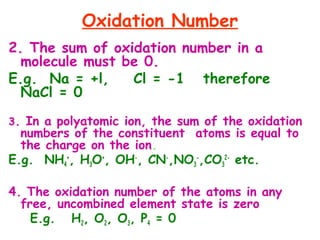
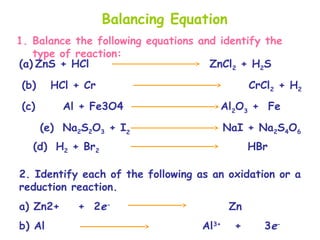
This document provides information on chemical equations and oxidation-reduction reactions. It defines key concepts like oxidation, reduction, oxidizing agents, reducing agents and oxidation numbers. Examples of different types of chemical reactions like combination, decomposition, displacement and combustion are outlined. Steps for writing and balancing chemical equations are described. Oxidation-reduction reactions are explained along with biological examples of electron transfer processes. Specific equations are given and identified as oxidation or reduction reactions.