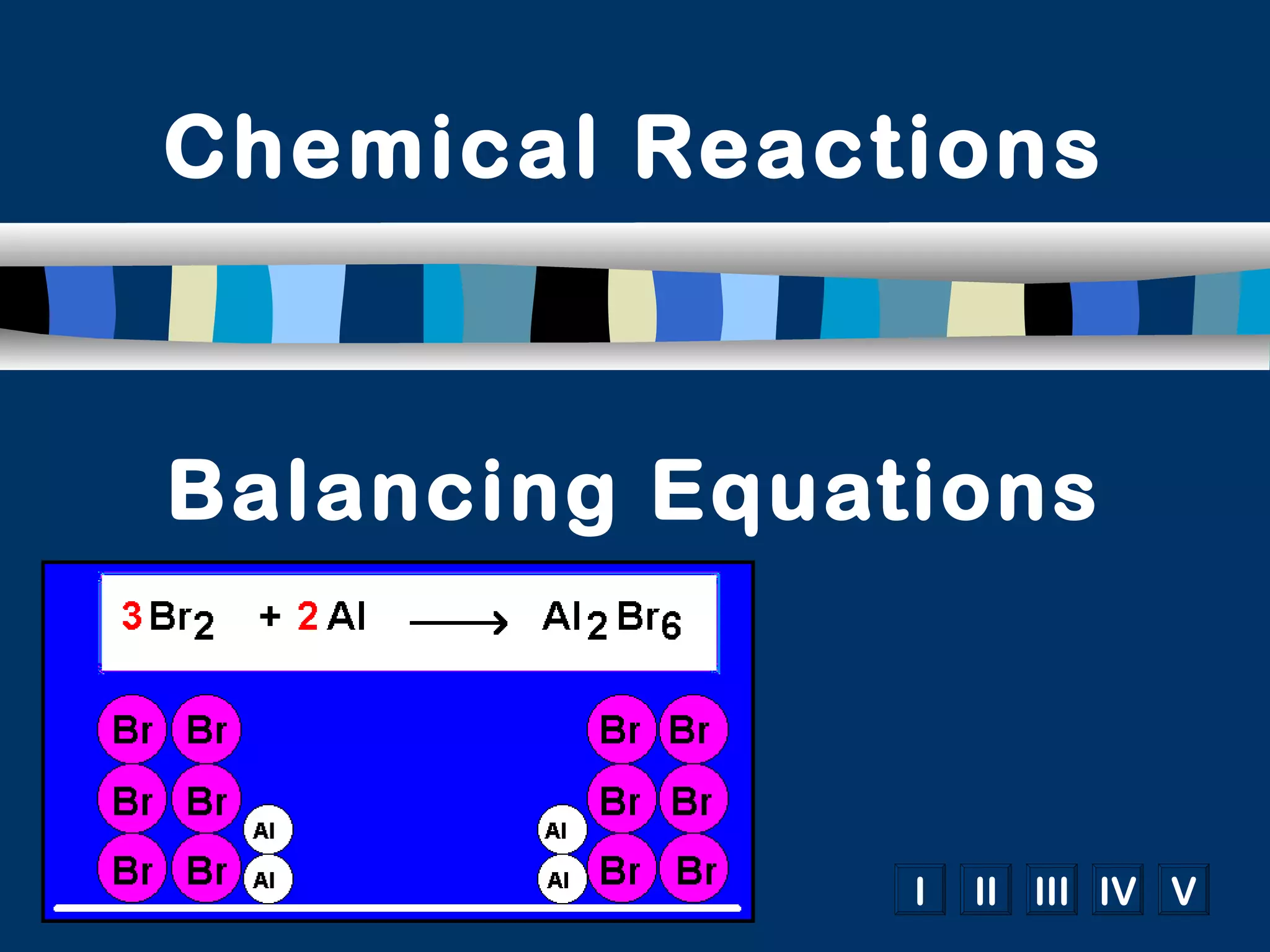
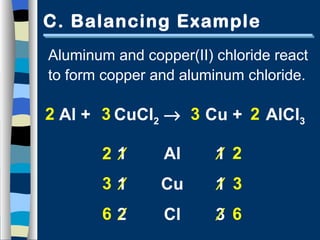
The document discusses balancing chemical equations in 5 steps: 1) write the unbalanced equation, 2) count atoms on each side, 3) add coefficients to make atom numbers equal, 4) reduce coefficients to the lowest ratio, and 5) double check atom balance. It provides tips such as balancing one element at a time and updating all atom counts after each coefficient addition. An example reaction of aluminum and copper(II) chloride is given and balanced according to the steps.