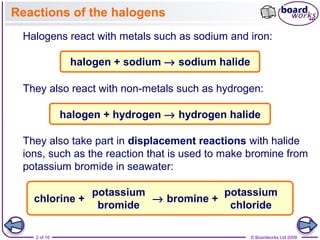
1. The document discusses the reactions of halogens, including their reactions with metals like sodium and iron, and with non-metals like hydrogen.
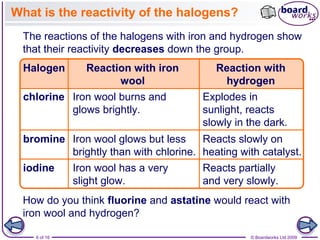
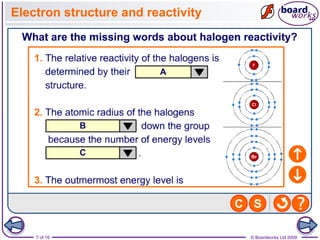
2. It explains that the reactivity of the halogens decreases down the group, with fluorine being the most reactive and reacting violently with iron wool and hydrogen, while iodine reacts only slowly.
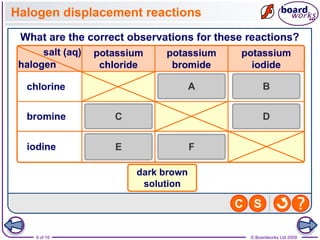
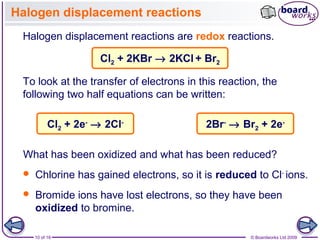
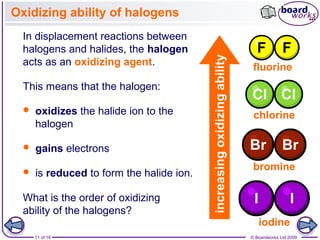
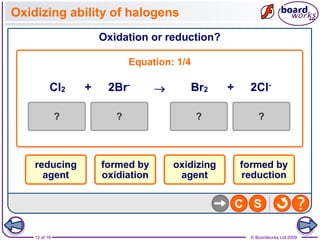
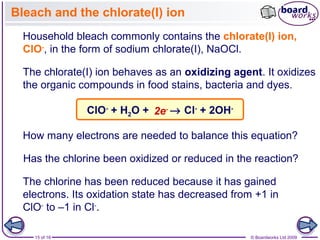
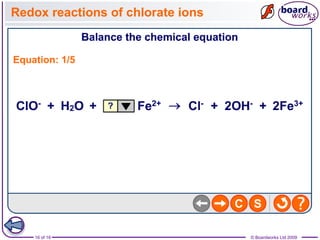
3. Halogen displacement reactions are described as redox reactions, where the more reactive halogen oxidizes the halide ion, gaining electrons itself and being reduced to form halide ions.