1) The document discusses naming compounds and balancing chemical equations using oxidation numbers. It provides examples of naming metal and non-metal compounds as well as oxide compounds.
2) Methods for determining oxidation numbers from compound names and formulas are explained. Examples are given for nitrate compounds and working out formulas from compound names.
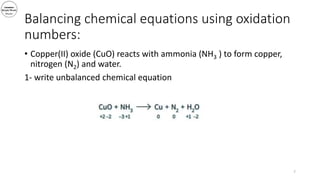
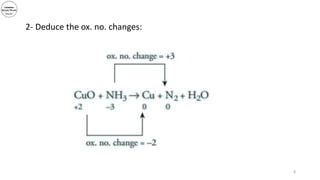
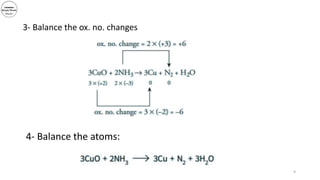
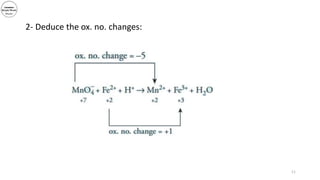
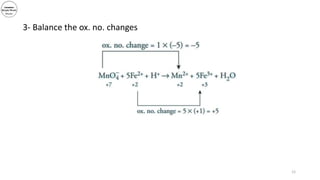
3) The document demonstrates balancing chemical equations using oxidation numbers, giving examples of copper (II) oxide reacting with ammonia and manganate (VII) ions reacting with iron (II) ions.