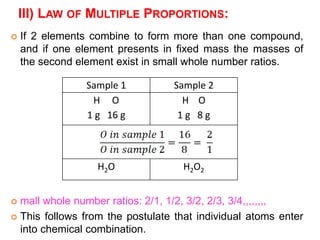
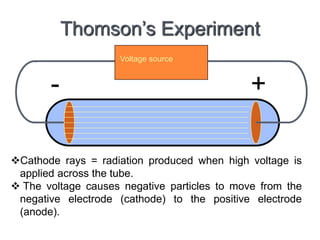
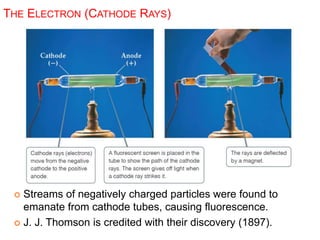
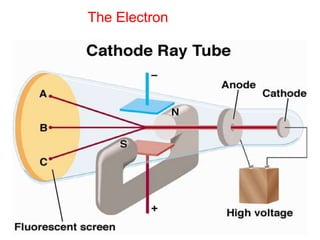
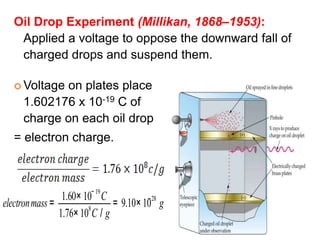
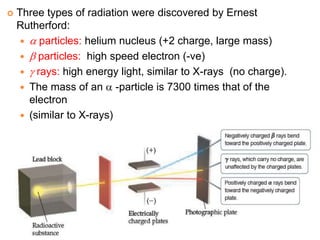
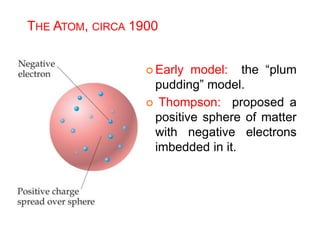
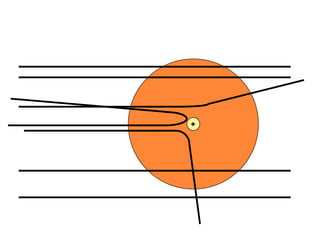
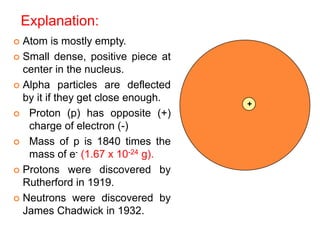
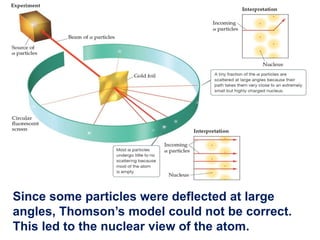
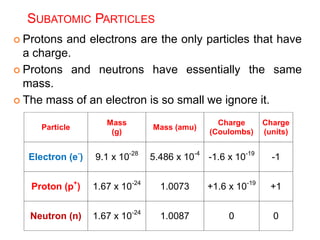
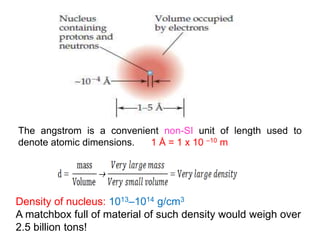
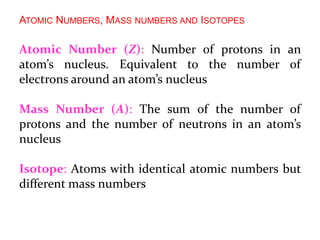
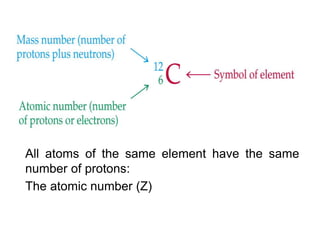
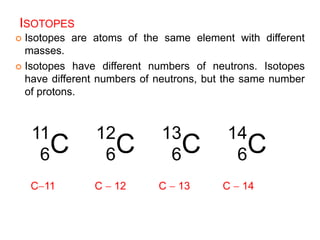
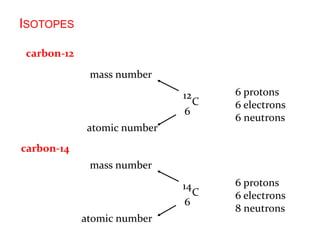
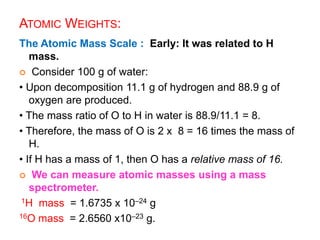
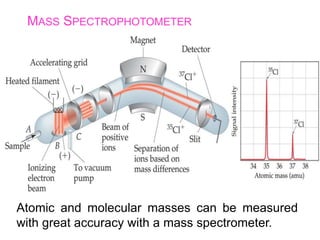
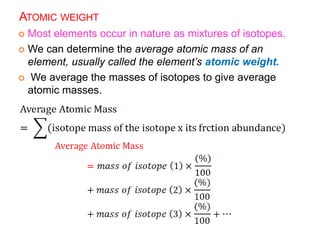
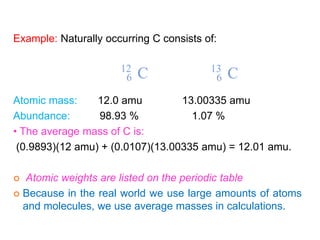
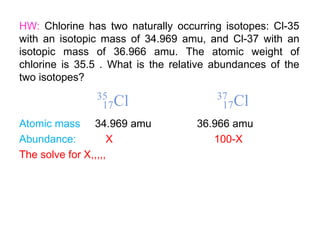
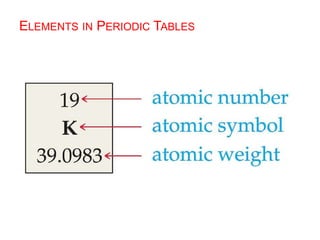
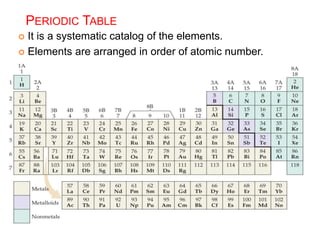
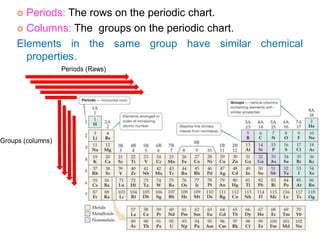
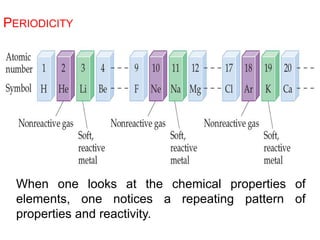
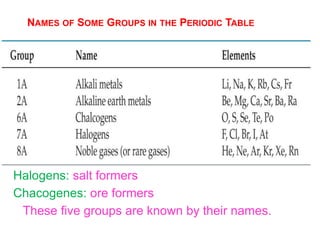
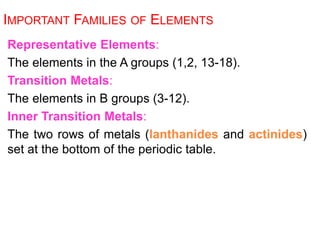
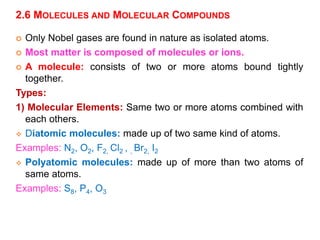
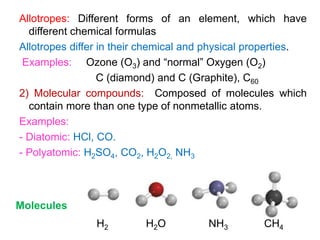
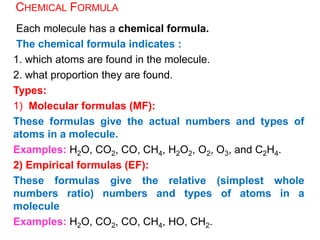
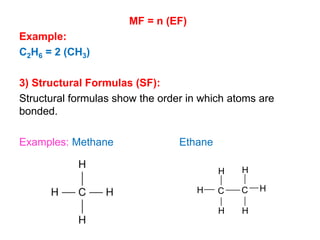
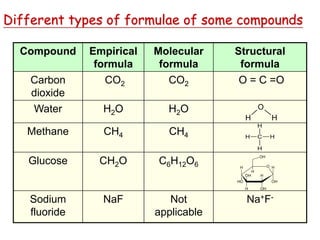
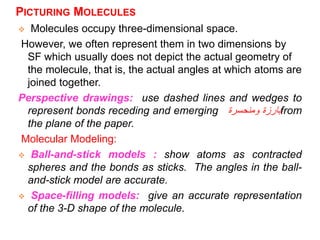
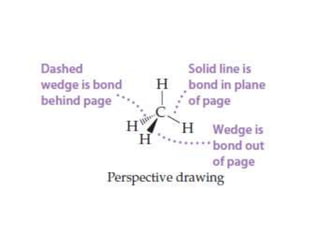
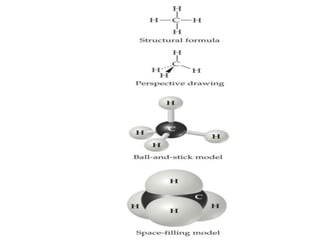
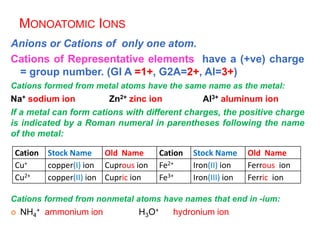
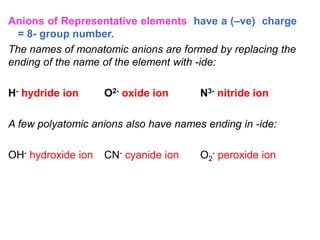
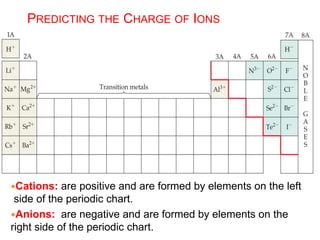
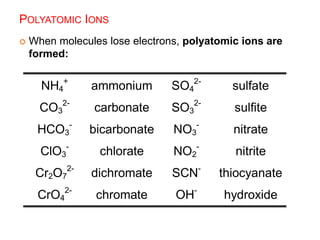
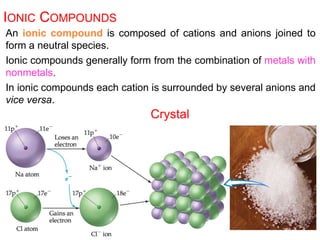
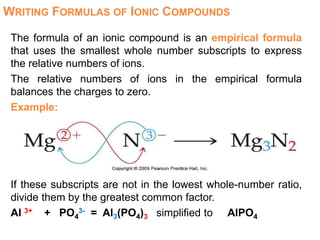
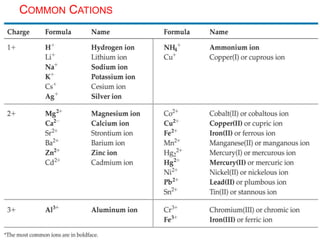
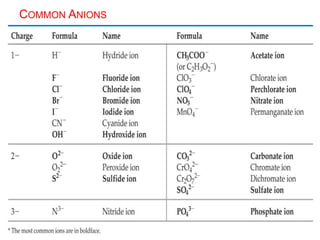
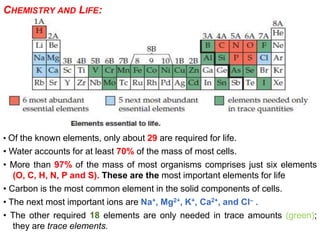
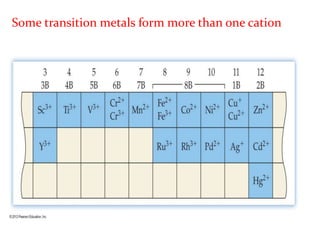
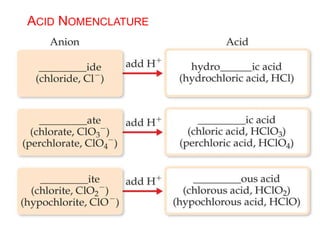
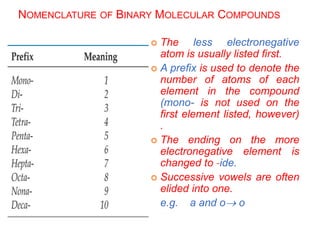
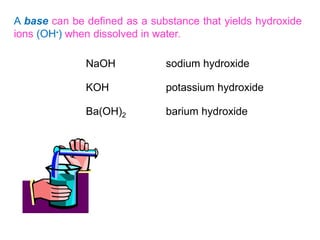
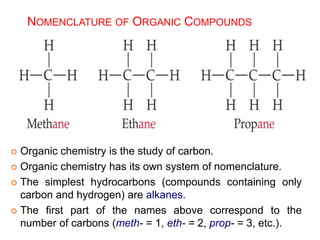
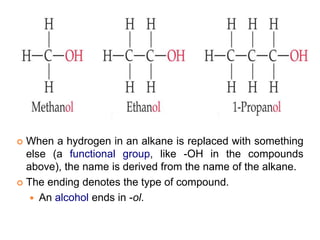
This document provides an overview of atomic structure and the development of atomic theory. It discusses early Greek philosophers' idea that matter is made of tiny particles and the contributions of scientists like Dalton, Thomson, Rutherford, and Millikan. Dalton established the basic atomic theory that all matter is made of atoms that cannot be divided. Rutherford's gold foil experiment led to the discovery of the nuclear model of the atom with a small, dense nucleus. The document also covers atomic and mass numbers, isotopes, atomic weights, and the periodic table.