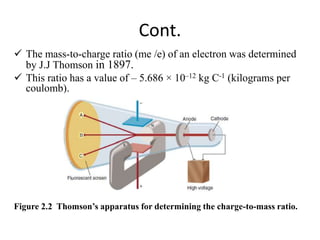
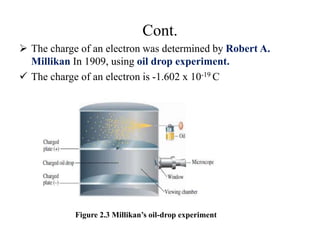
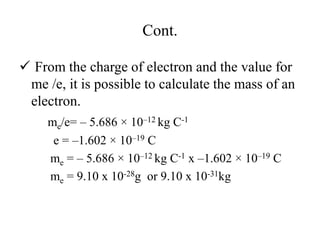
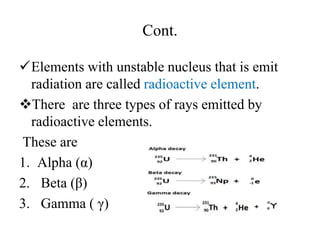
1. The document discusses the historical development of atomic theory from ancient Greek philosophers to modern atomic theory. It describes key experiments and discoveries such as cathode rays, electrons, radioactivity, and the discovery of the nucleus and neutron.
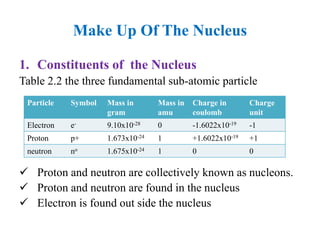
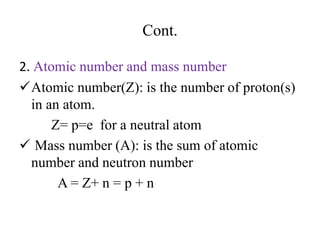
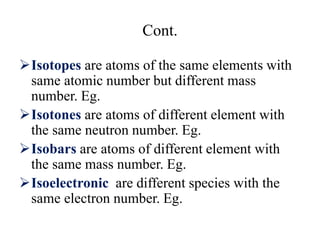
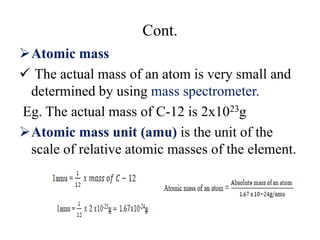
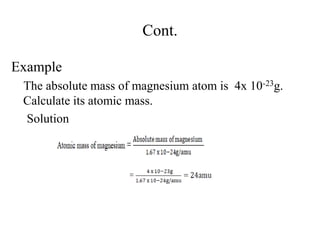
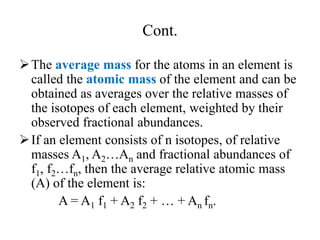
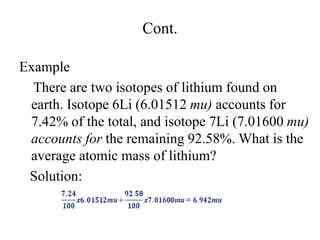
2. The makeup of atoms and the nucleus are explained. Atoms are made up of protons, neutrons, and electrons. The nucleus contains protons and neutrons. Isotopes, atomic number, mass number, and average atomic mass are defined.
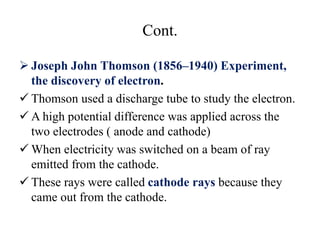
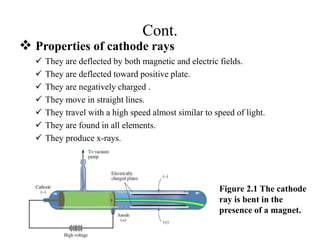
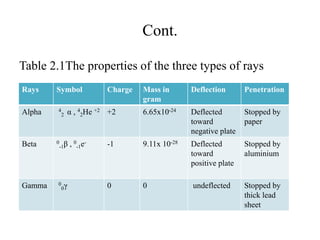
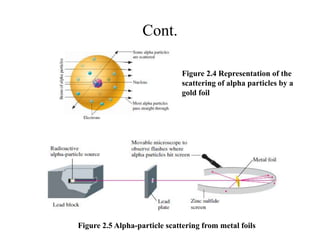
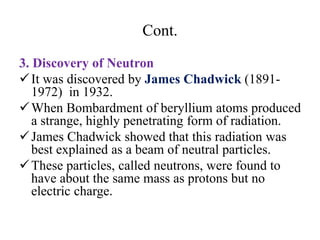
3. Early experiments that helped characterize the atom are described, including cathode ray experiments, discovery of the electron, discovery of the nucleus via alpha scattering experiments, and discovery of the neutron.