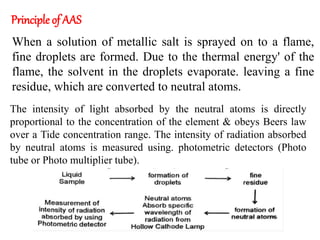
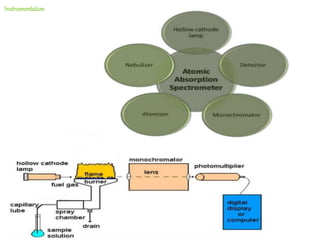
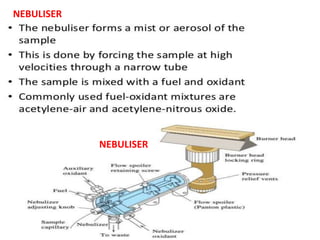
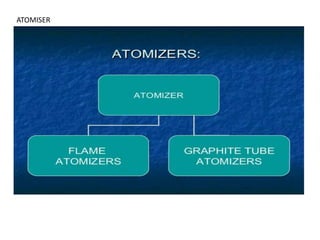
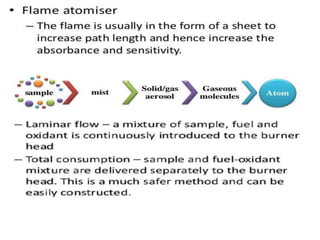
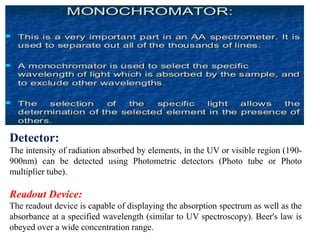
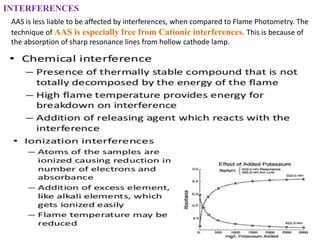
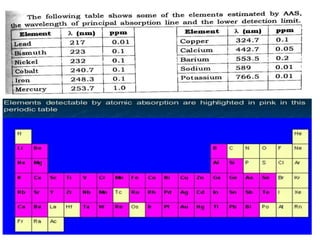
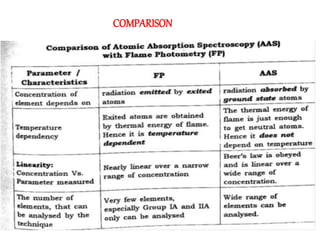
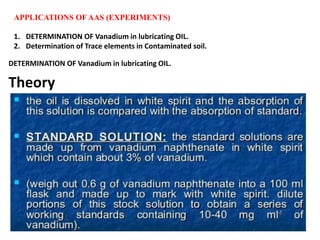
Atomic absorption spectroscopy (AAS) is a technique used to determine the concentration of metallic elements in solutions. It works by vaporizing the sample into neutral atoms and measuring the absorption of light from a hollow cathode lamp at a specific wavelength. The intensity of absorbed light is directly proportional to the concentration of the element. AAS can detect low ppm levels of elements and is used to analyze biological fluids, foods, soils, and other materials. It has advantages over flame photometry in being less susceptible to cationic interferences. Examples of applications include determining levels of copper, zinc, and lead in various products and samples.