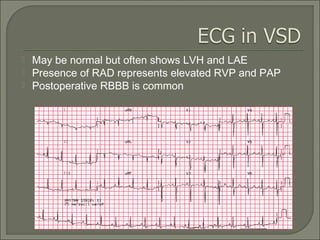
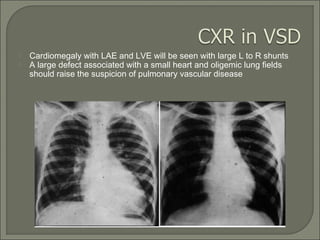
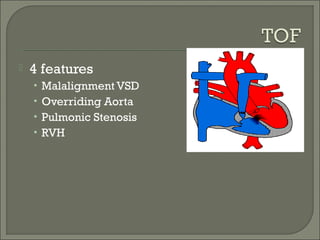
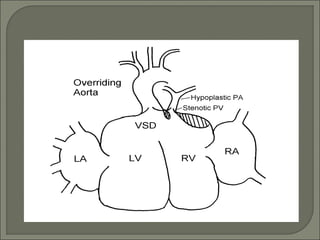
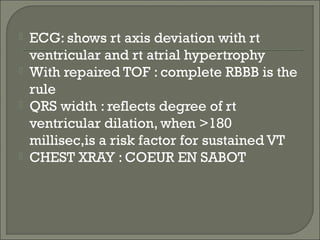
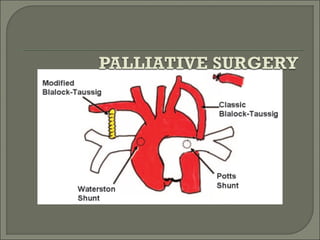
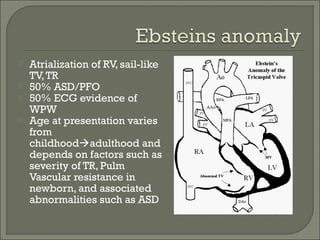
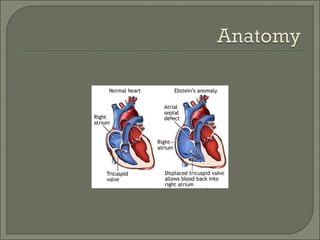
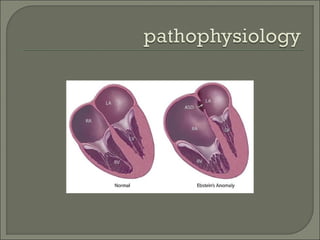
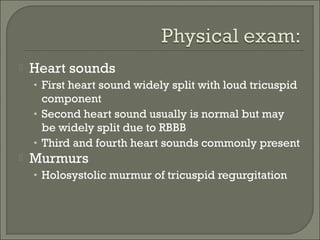
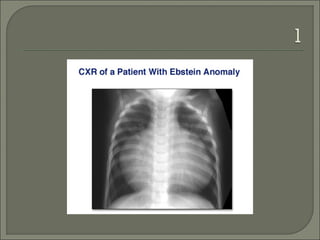
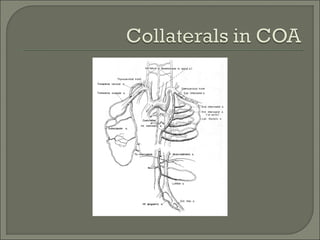
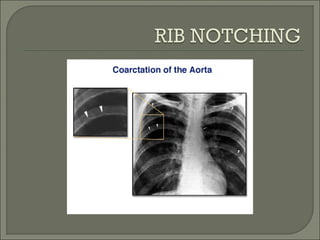
This document discusses congenital heart disease in adults. It notes that 1 million adults in the US have congenital heart disease, with 20,000 more reaching adulthood each year due to increased survival of children with CHD. Common adult presentations of CHD include effort dyspnea, atrial fibrillation, and right heart failure. The document reviews the pathophysiology, clinical features, diagnostic evaluation, and management of various CHD lesions that may present in adulthood, such as atrial septal defects, ventricular septal defects, patent ductus arteriosus, tetralogy of Fallot, Ebstein's anomaly, and coarctation of the aorta. Surgical and percutaneous interventions are discussed