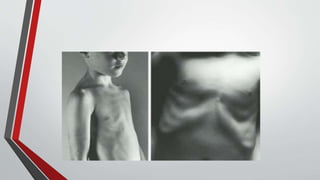
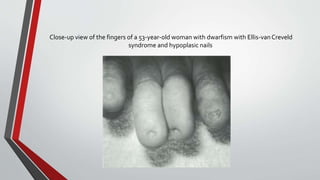
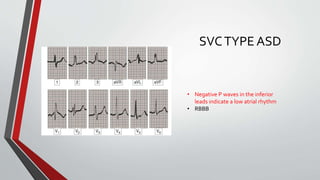
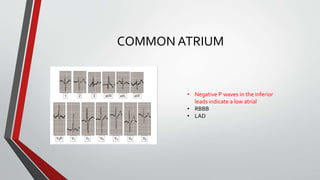
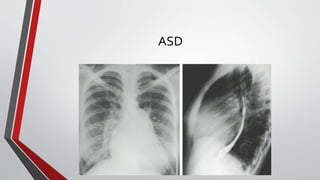
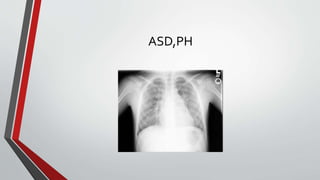
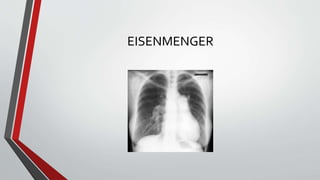
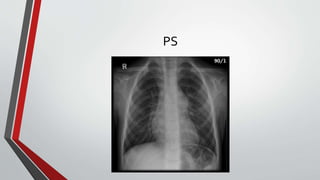
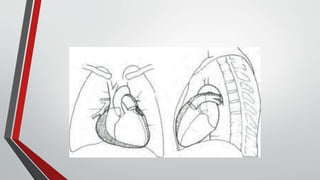
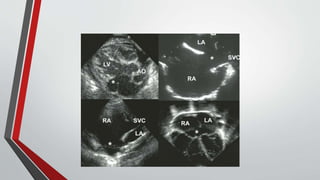
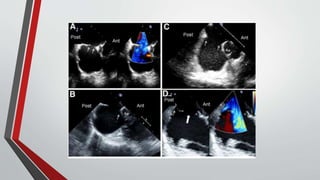
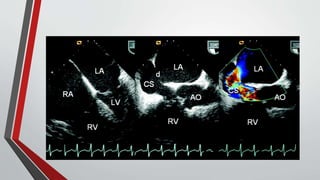
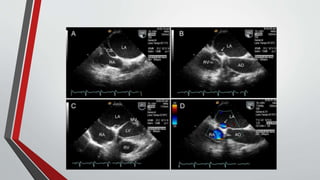
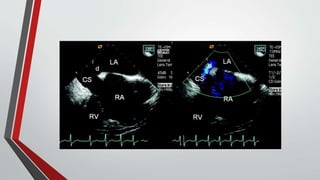
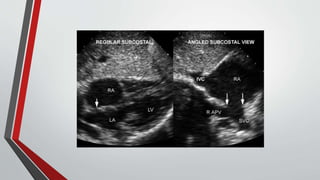
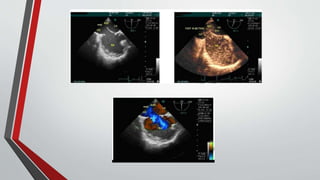
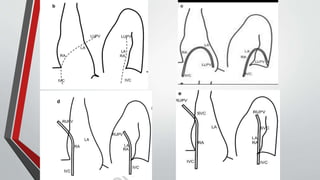
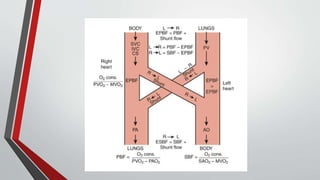
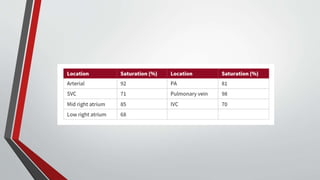
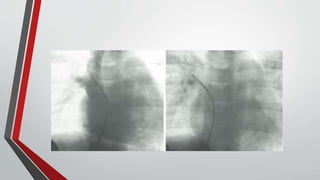
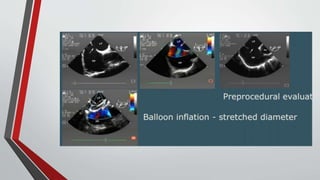
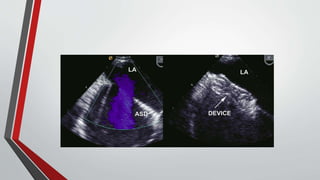
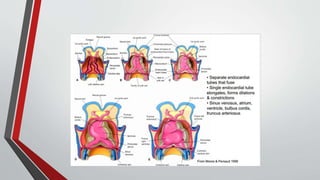
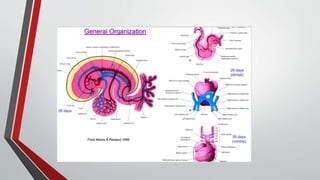
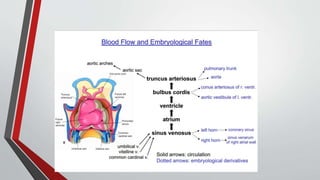
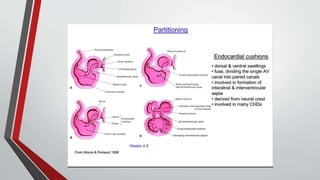
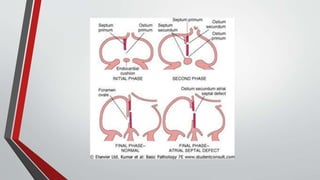
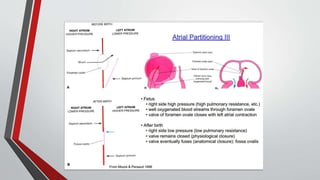
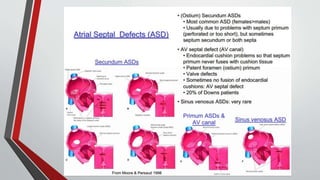
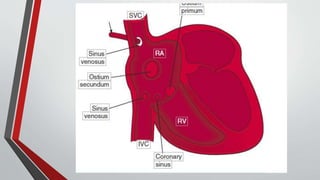
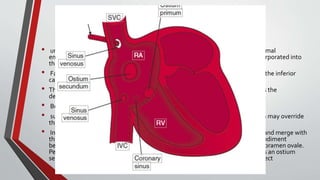
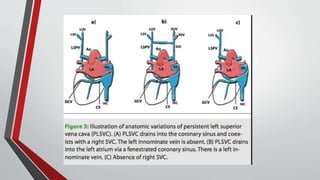
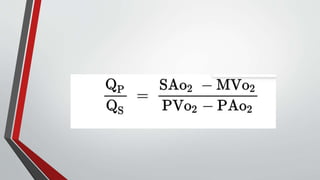Leonardo da Vinci was the first to document a true atrial septal defect in his writings. The document then describes the normal process of atrial septation during embryonic development and how defects can occur if this process is disrupted. It classifies the main types of atrial septal defects and describes the characteristics, causes and clinical implications of each. Ostium secundum defects are the most common type while sinus venosus and coronary sinus defects are less common. The pathophysiology of left-to-right shunting with an atrial septal defect is explained.
















![POINTS
• large ASD (pulmonary artery blood flow relative to systemic blood flow [Qp/Qs] >2.0:1.0) may cause congestive heart
failure and failure to thrive in an infant or child.
• An undetected ASD with a significant shunt (Qp/Qs >1.5:1.0) probably causes symptoms over time in adolescence or
adulthood, and symptomatic patients usually become progressively more physically limited as they age.
• Effort-related dyspnea is seen in approximately 30% of patients by the third decade and in more than 75% by the fifth
decade.
• Exercise intolerance on cardiopulmonary testing is even more common and reflects the fact that such patients often
do not know what “normal” feels like.
• Supraventricular arrhythmias (atrial fibrillation or flutter) and right-sided heart failure develop by 40 years of age in
approximately 10% of patients and become more prevalent with aging.
• Paradoxical embolism resulting in a transient ischemic attack or stroke can call attention to the diagnosis.
• The development of pulmonary hypertension, although probably not as common as originally thought, can occur at
an early age.
• If pulmonary hypertension is severe, a second causative diagnosis should be sought.
• Life expectancy is clearly reduced in patients with an ASD, although not as severely as was quoted in earlier papers
because only patients with large ASDs were reported.](https://image.slidesharecdn.com/atrialseptaldefect-141103103854-conversion-gate01/85/Atrial-septal-defect-28-320.jpg)