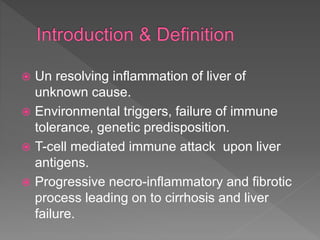
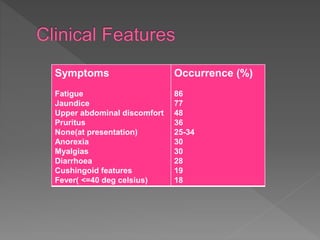
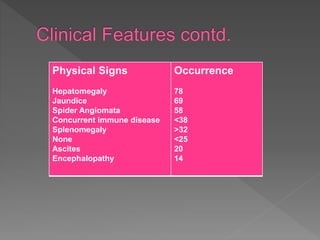
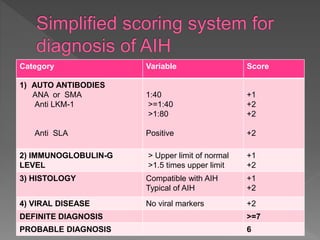
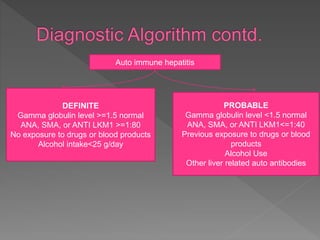
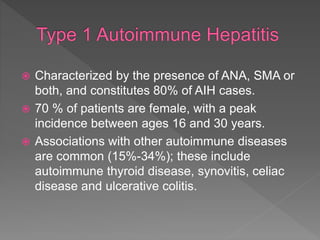
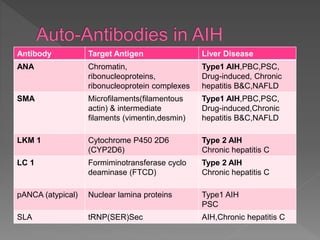
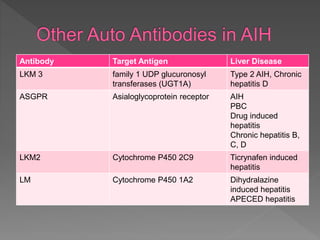
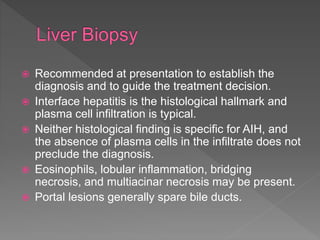
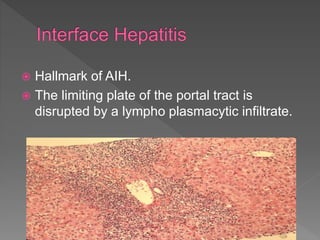
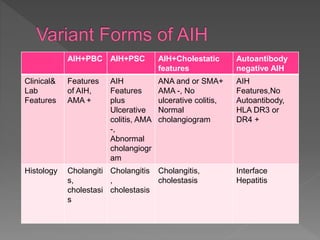
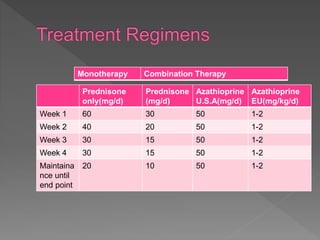
This document summarizes information about autoimmune hepatitis (AIH), including:
- It is a T-cell mediated immune attack on the liver that causes progressive damage and can lead to cirrhosis.
- Two main types (type 1 and type 2) are distinguished by their associated autoantibodies.
- Women are affected more often than men. Treatment involves immunosuppression with glucocorticoids alone or in combination with azathioprine to induce remission. Response to treatment and long term outcomes depend on disease severity at presentation.