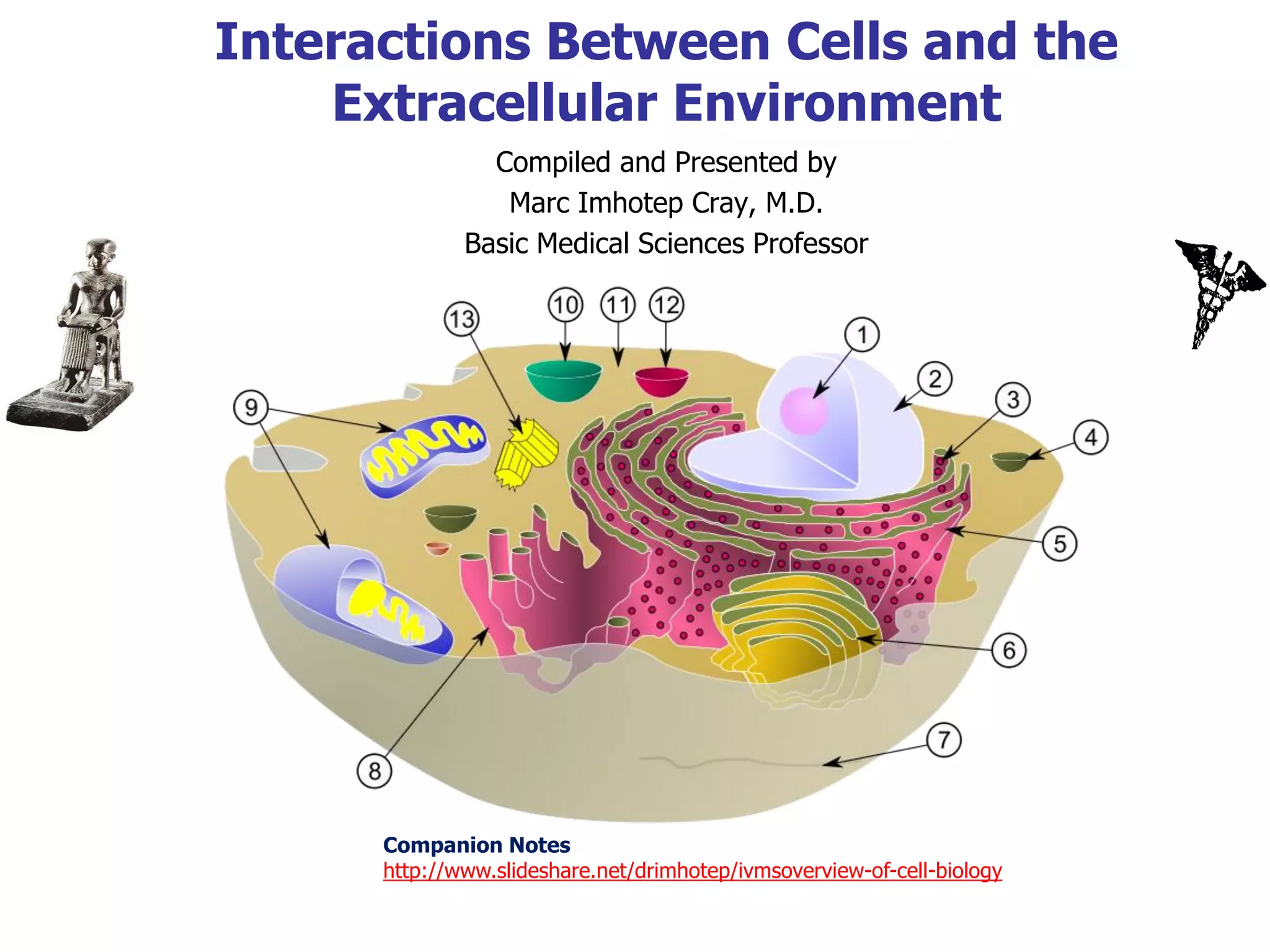
The document discusses interactions between cells and the extracellular environment. It describes the extracellular matrix as consisting of collagen, elastin, and ground substance containing interstitial fluid. It then covers various mechanisms of transport across the plasma membrane including diffusion, osmosis, carrier-mediated transport, and bulk transport. It also addresses concepts such as membrane potential, equilibrium potential, and cell signaling.
![Osmosis
Net diffusion of H20 across a
selectively permeable membrane.
Movement of H20 from a high[H20]
to lower [H20] until equilibrium is
reached.
2 requirements for osmosis:
Must be difference in [solute] on the
2 sides of the membrane.
Membrane must be impermeable to
the solute.
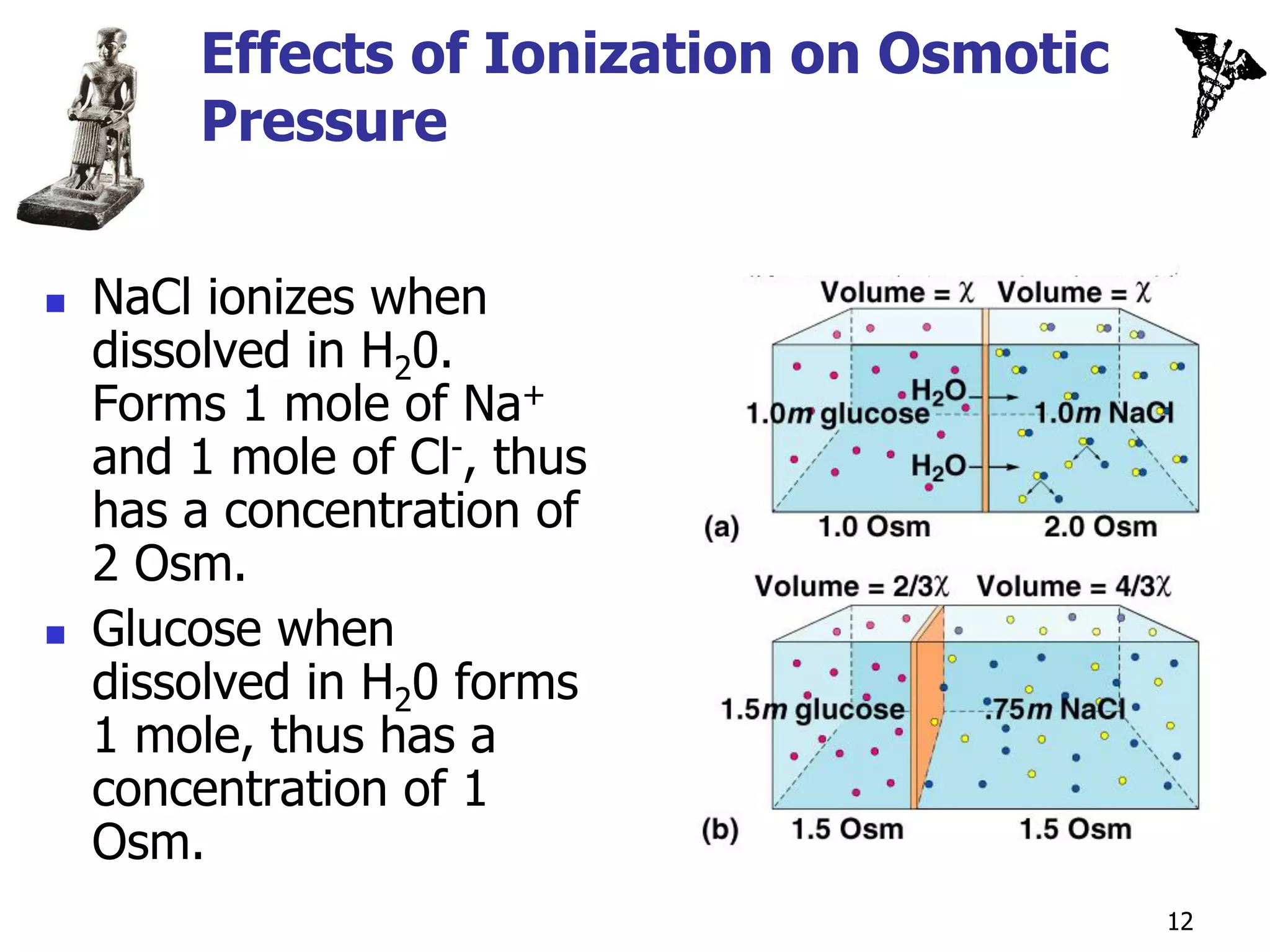
Osmotically active solutes:
Solutes that cannot pass freely
through the membrane.
8](https://image.slidesharecdn.com/ivms-interactionsbetweencellsandtheextracellularenvironment-120611175235-phpapp01/75/IVMS-Interactions-Between-Cells-and-the-Extracellular-Environment-8-2048.jpg)
![Effects of Osmosis
H20 moves by osmosis into the lower [H20]
until equilibrium is reached (270 g/l glucose).
Osmosis ceases when concentrations are
equal on both sides of the membrane.
9](https://image.slidesharecdn.com/ivms-interactionsbetweencellsandtheextracellularenvironment-120611175235-phpapp01/75/IVMS-Interactions-Between-Cells-and-the-Extracellular-Environment-9-2048.jpg)
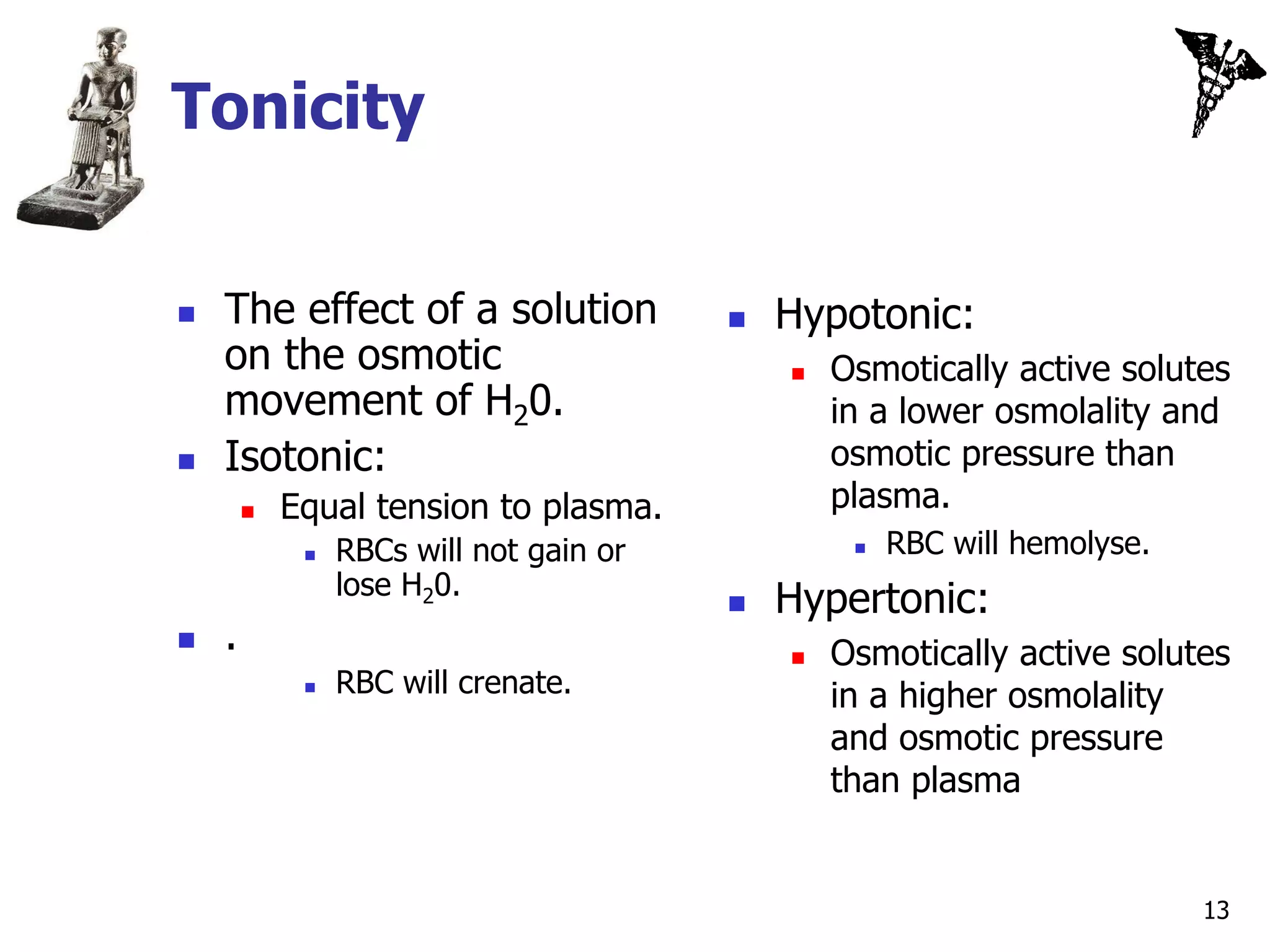
![Osmotic Pressure
The force that would have to be exerted to prevent
osmosis.
The greater the [solute] of solution, the > the osmotic
pressure.
Indicates how strongly the solution “draws” H20 into it by
osmosis.
10](https://image.slidesharecdn.com/ivms-interactionsbetweencellsandtheextracellularenvironment-120611175235-phpapp01/75/IVMS-Interactions-Between-Cells-and-the-Extracellular-Environment-10-2048.jpg)
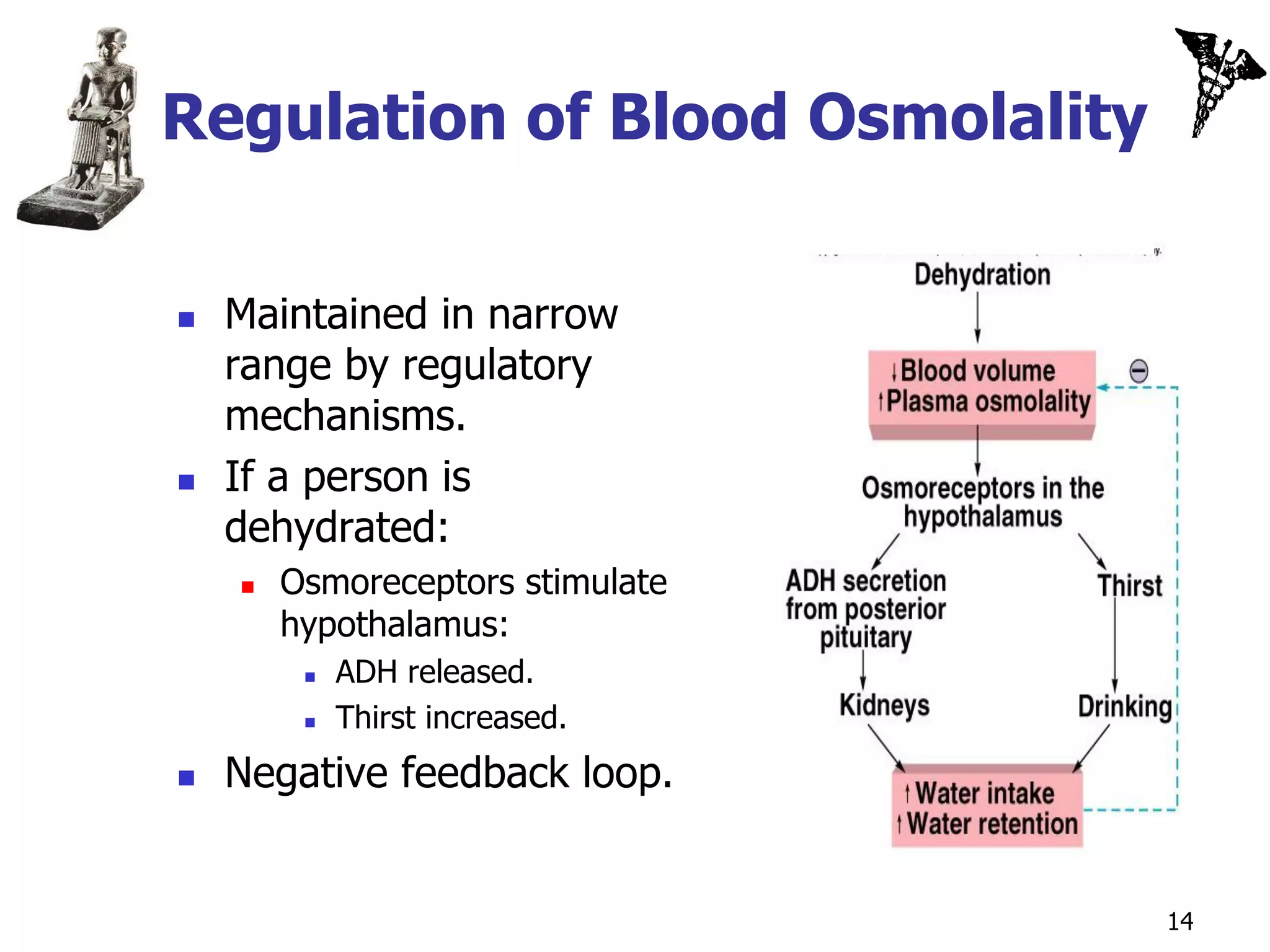
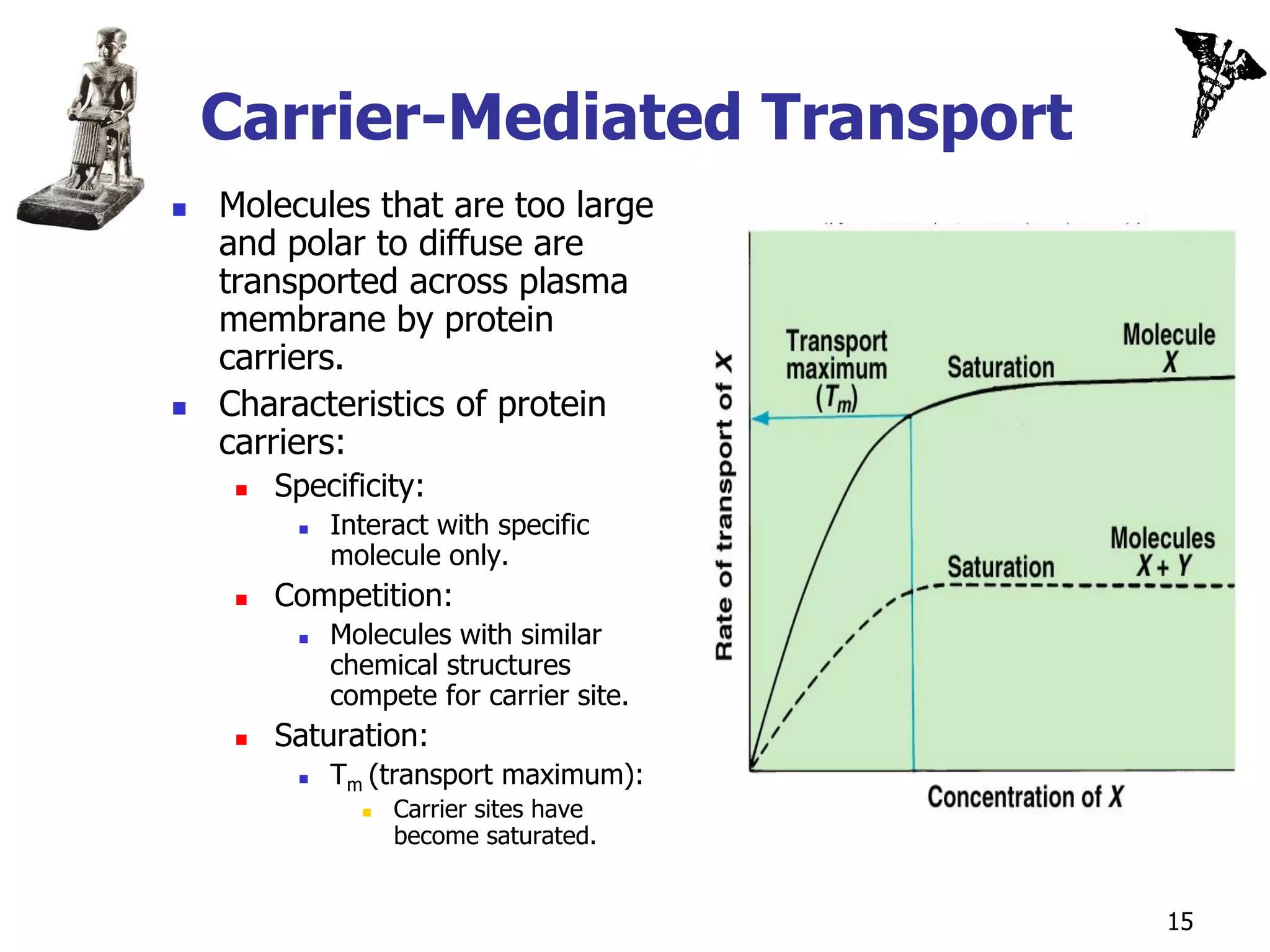
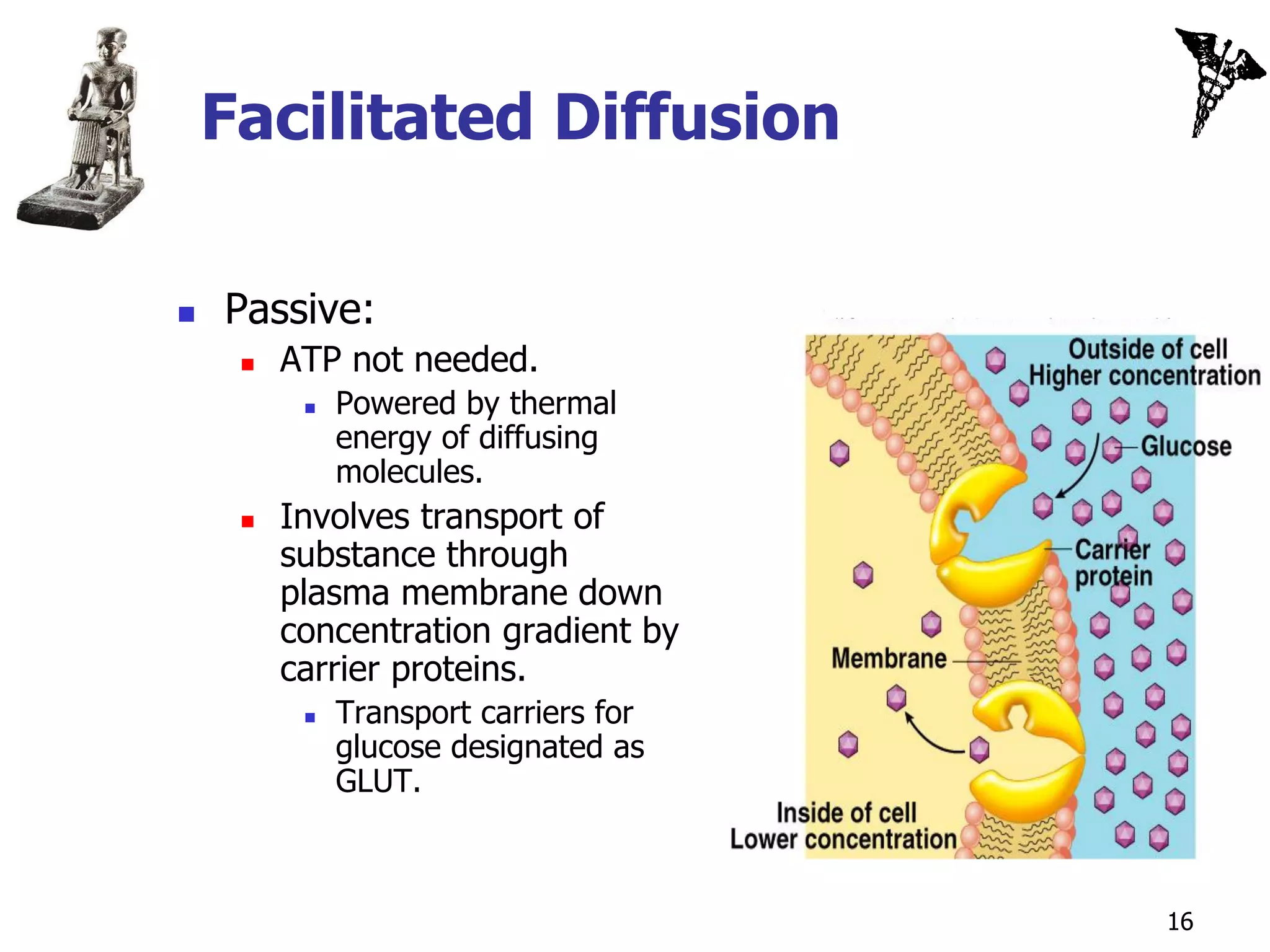
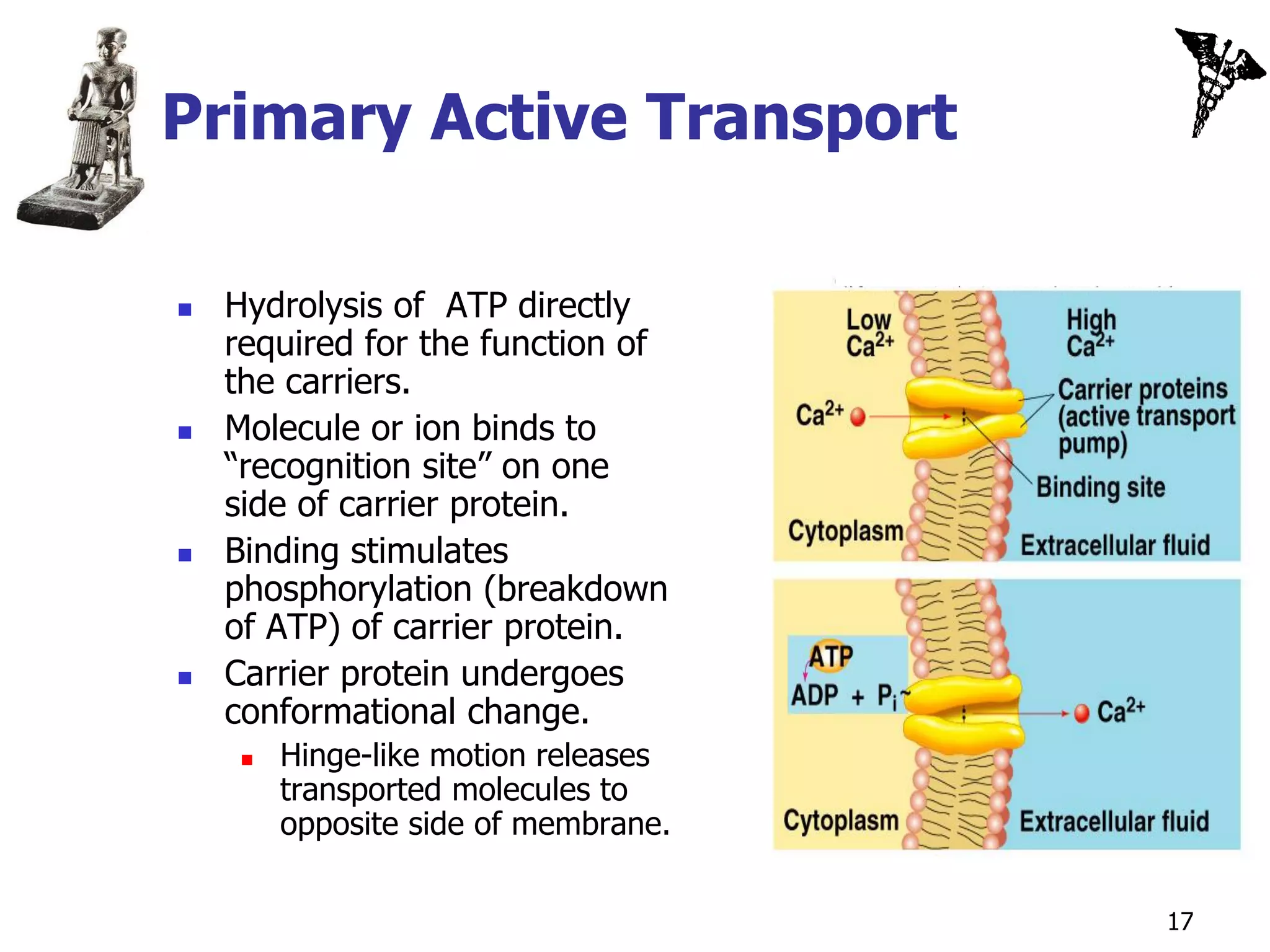
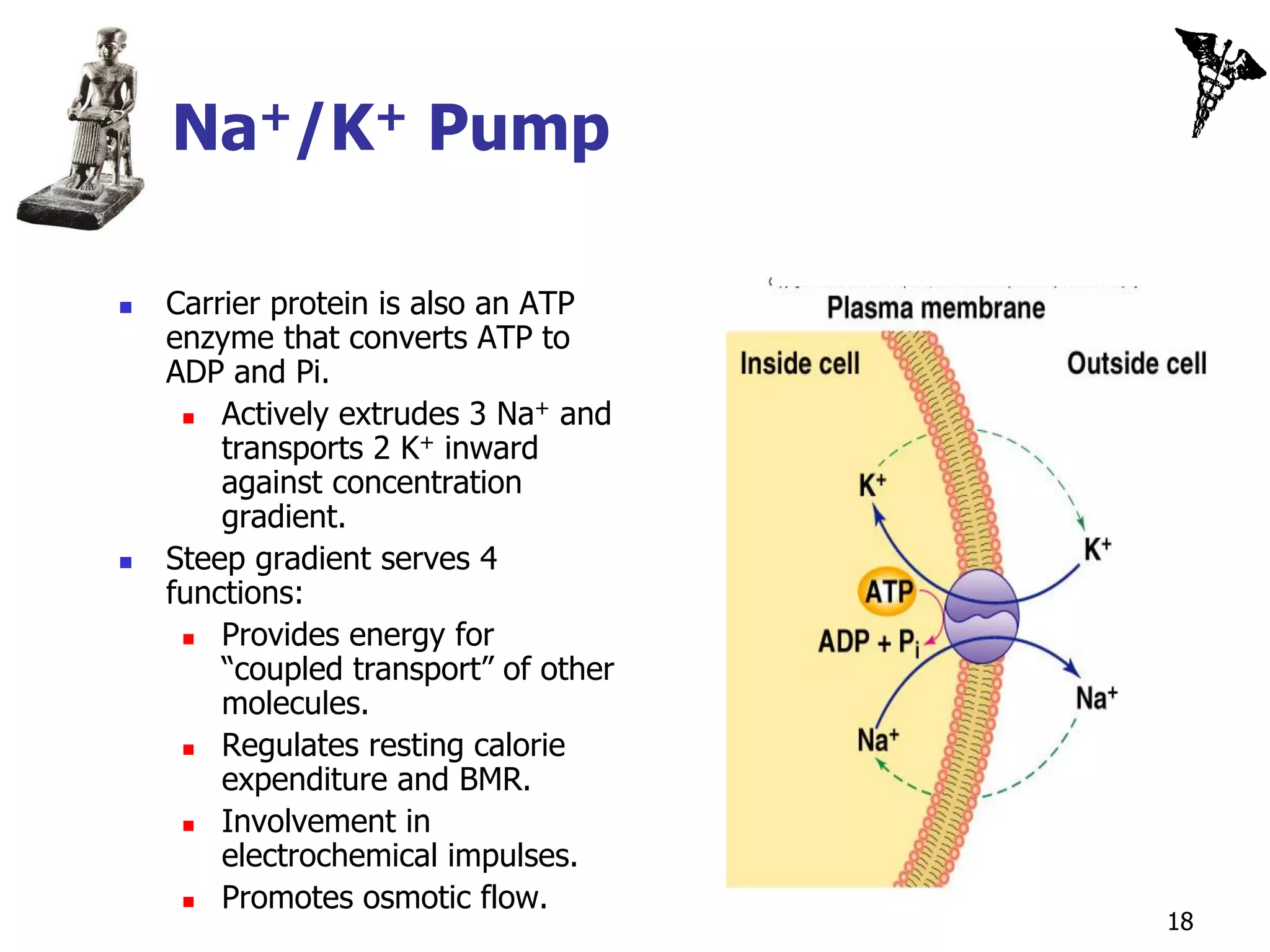
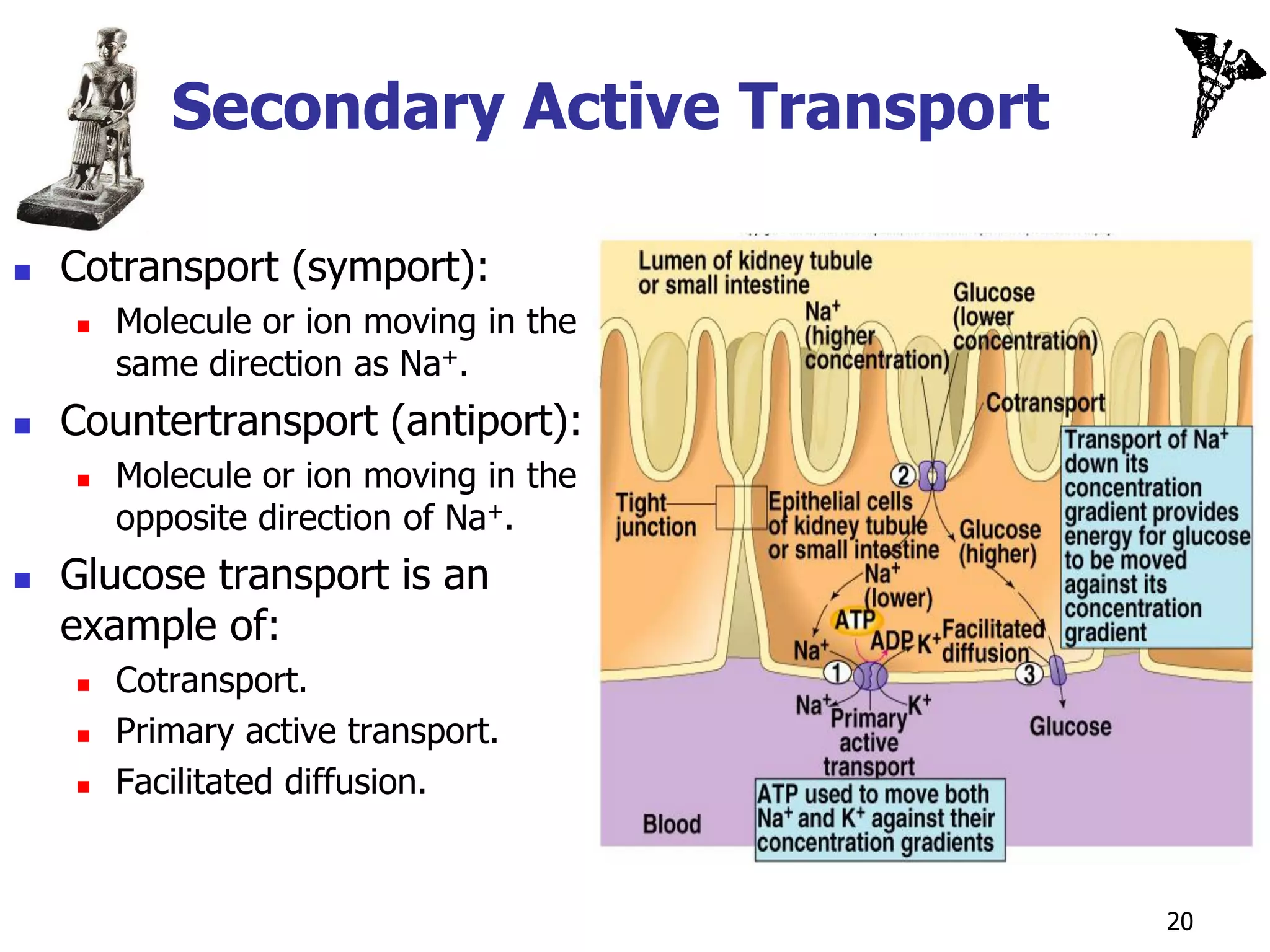
![Secondary Active Transport
Coupled transport.
Energy needed for “uphill” movement obtained
from “downhill” transport of Na+.
Hydrolysis of ATP by Na+/K+ pump required
indirectly to maintain [Na+] gradient.
19](https://image.slidesharecdn.com/ivms-interactionsbetweencellsandtheextracellularenvironment-120611175235-phpapp01/75/IVMS-Interactions-Between-Cells-and-the-Extracellular-Environment-19-2048.jpg)
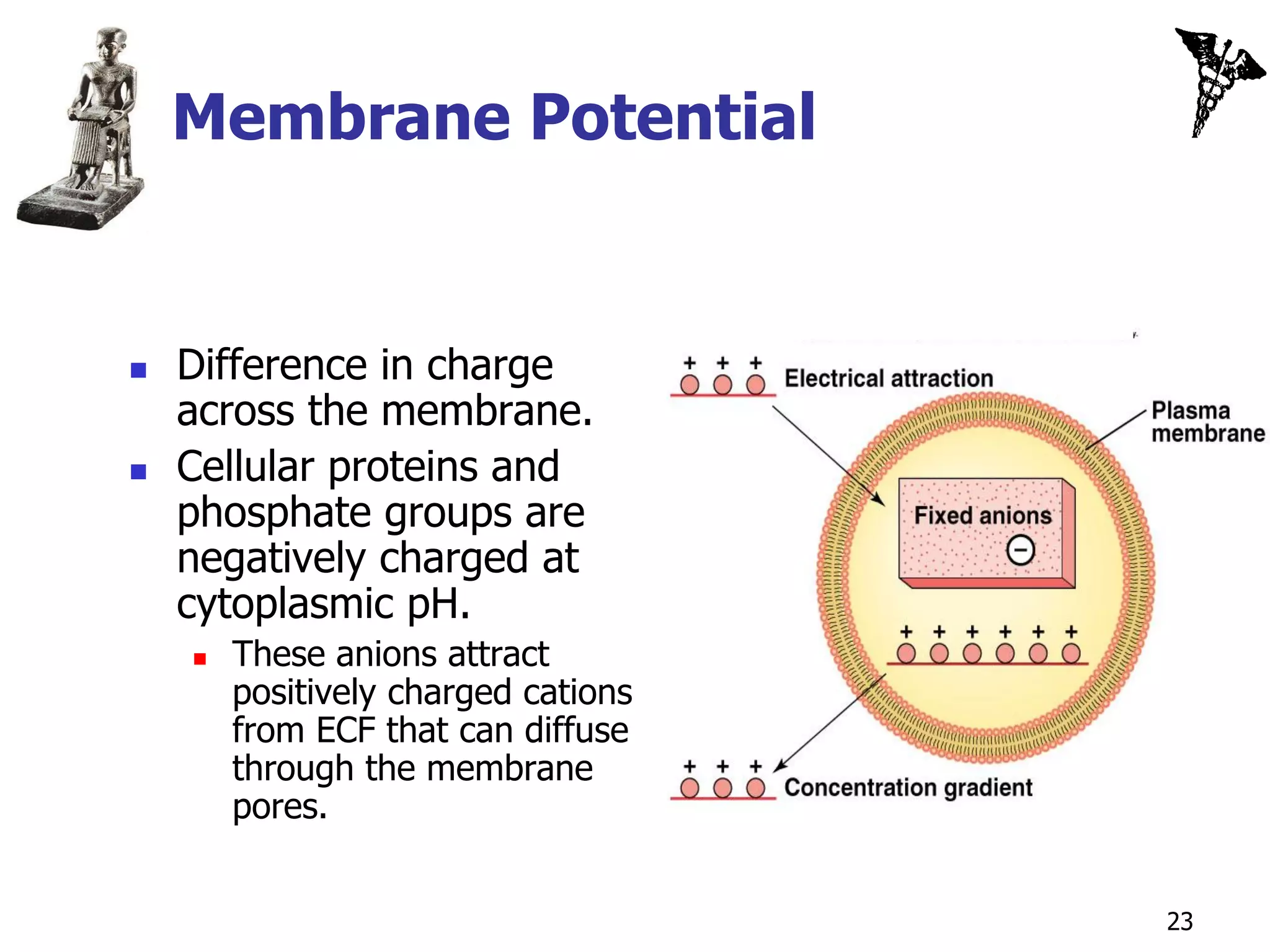
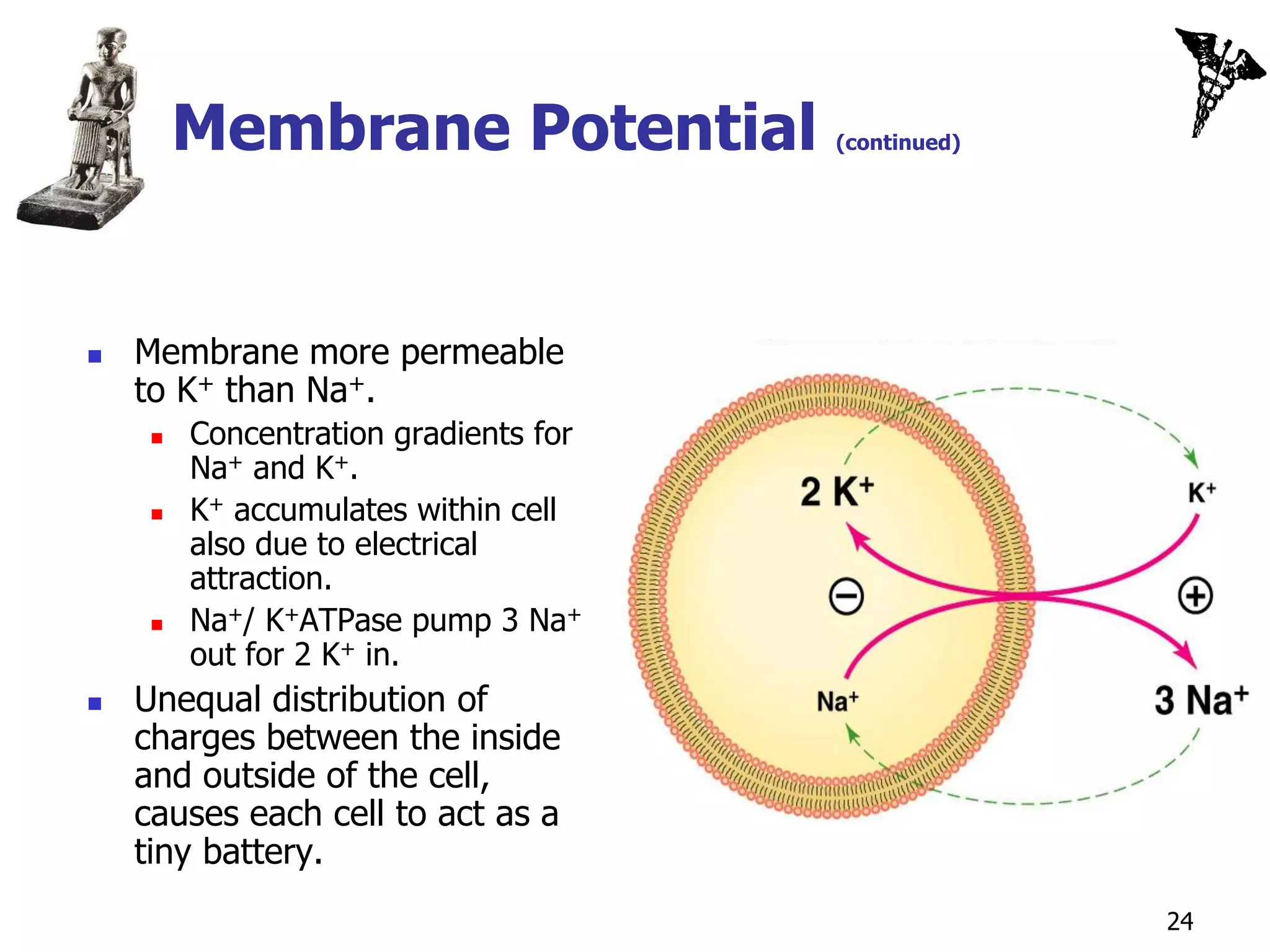
![Equilibrium Potentials
Theoretical voltage produced across
the membrane if only 1 ion could
diffuse through the membrane.
If membrane only permeable to K+,
K+ diffuses until [K+] is at
equilibrium.
Force of electrical attraction and
diffusion are = and opposite.
At equilibrium, inside of the cell
membrane would have a higher
[negative charges] than the
outside.
Potential difference:
Magnitude of difference in charge
on the 2 sides of the membrane.
25](https://image.slidesharecdn.com/ivms-interactionsbetweencellsandtheextracellularenvironment-120611175235-phpapp01/75/IVMS-Interactions-Between-Cells-and-the-Extracellular-Environment-25-2048.jpg)
![Nernst Equation
Allows theoretical membrane potential to be
calculated for particular ion.
Membrane potential that would exactly balance the
diffusion gradient and prevent the net movement
of a particular ion.
Value depends on the ratio of [ion] on the 2 sides
of the membrane.
Ex = 61 log [Xo]
z [Xi]
Equilibrium potential for K+ = - 90 mV.
Equilibrium potential for Na+ = + 60 mV.
26](https://image.slidesharecdn.com/ivms-interactionsbetweencellsandtheextracellularenvironment-120611175235-phpapp01/75/IVMS-Interactions-Between-Cells-and-the-Extracellular-Environment-26-2048.jpg)