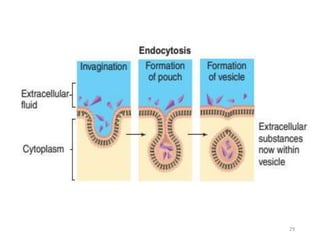
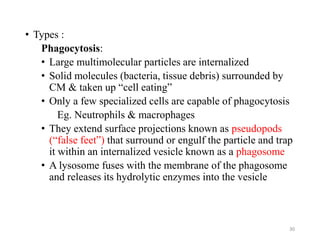
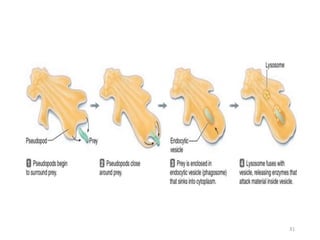
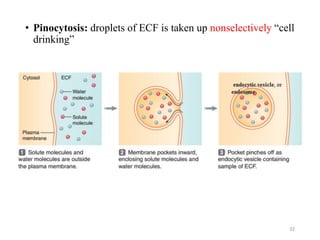
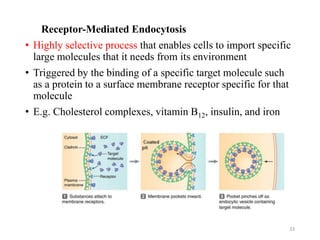
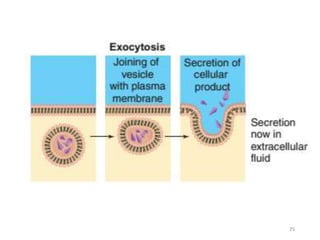
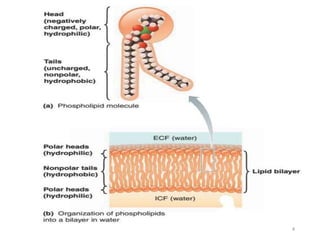
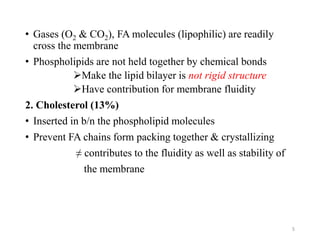
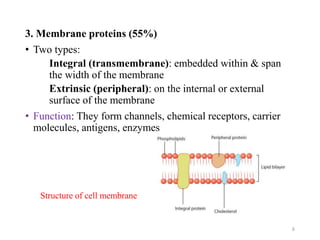
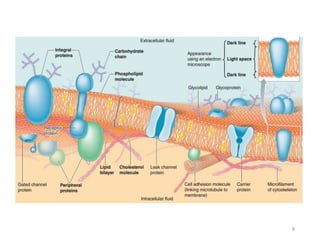
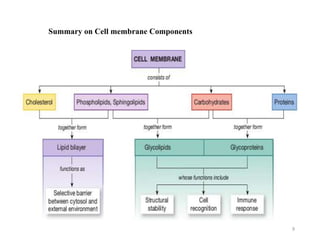
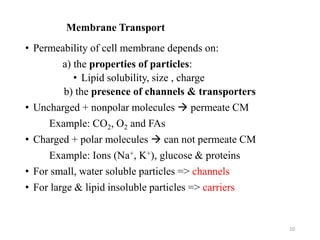
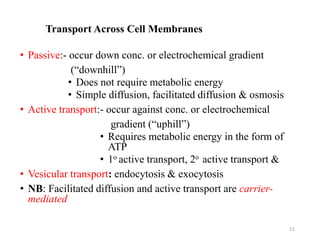
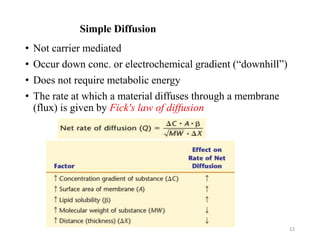
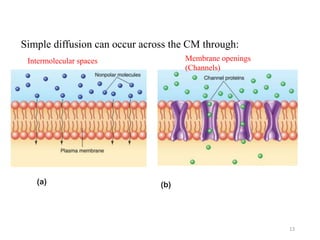
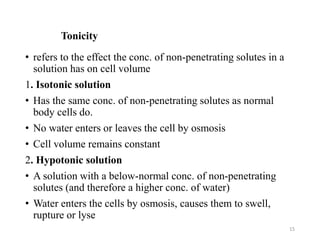
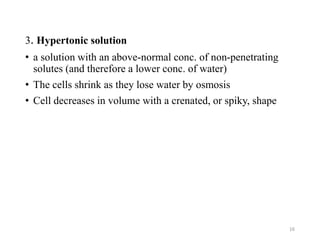
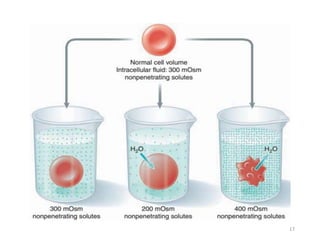
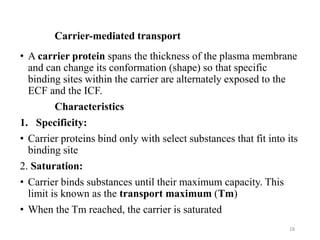
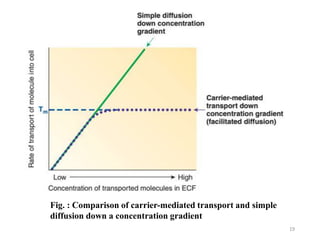
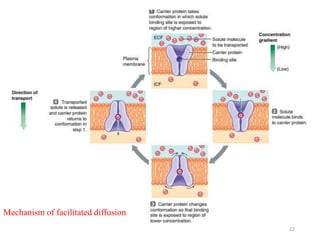
The document discusses membrane physiology, focusing on the structure and function of cell membranes, including their components such as phospholipids, cholesterol, proteins, and carbohydrates. It describes how substances permeate the cell membrane through mechanisms like passive and active transport, highlighting processes such as simple diffusion, facilitated diffusion, and vesicular transport. The document also covers concepts of tonicity and the roles of various transport proteins in maintaining cellular functionality.



![• Examples:
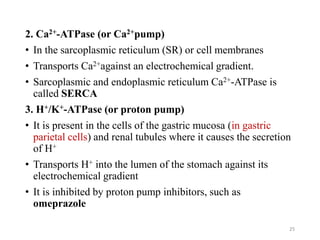
1. Na+/K+-ATPase (or Na+–K+ pump)
• In cell membranes
• Transports Na+ from ICF to ECF and K+ from ECF to ICF It
maintains low intracellular [Na+] and high intracellular [K+]
• Both Na+ and K+ are transported against their electrochemical
gradients
• The usual stoichiometry is 3 Na+/2 K+
• Produces net movement of positive charge out of the cell (an
electrogenic pump)
• This electrical potential is a basic requirement in nerve and
muscle fibers for transmitting electrical signals
• Specific inhibitors of Na+, K+-ATPase are the cardiac glycoside
drugs ouabain and digitalis
24](https://image.slidesharecdn.com/chapter2-membranephysiology-240721054846-49234232/85/Chapter-2-Membrane-physiology-power-pointspptx-24-320.jpg)