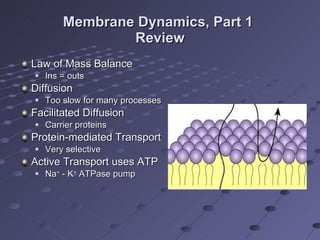
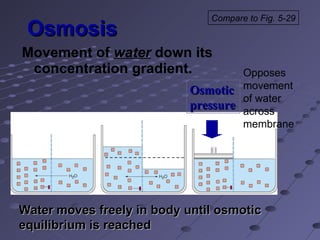
The document summarizes membrane structures and functions including methods of transport like vesicular transport, osmosis, and distribution of water and solutes. It discusses concepts such as membrane permeability, resting membrane potential, and electrical and chemical imbalances. Transepithelial transport and distribution of solutes in body fluid compartments are also covered.