Embed presentation
Downloaded 10 times



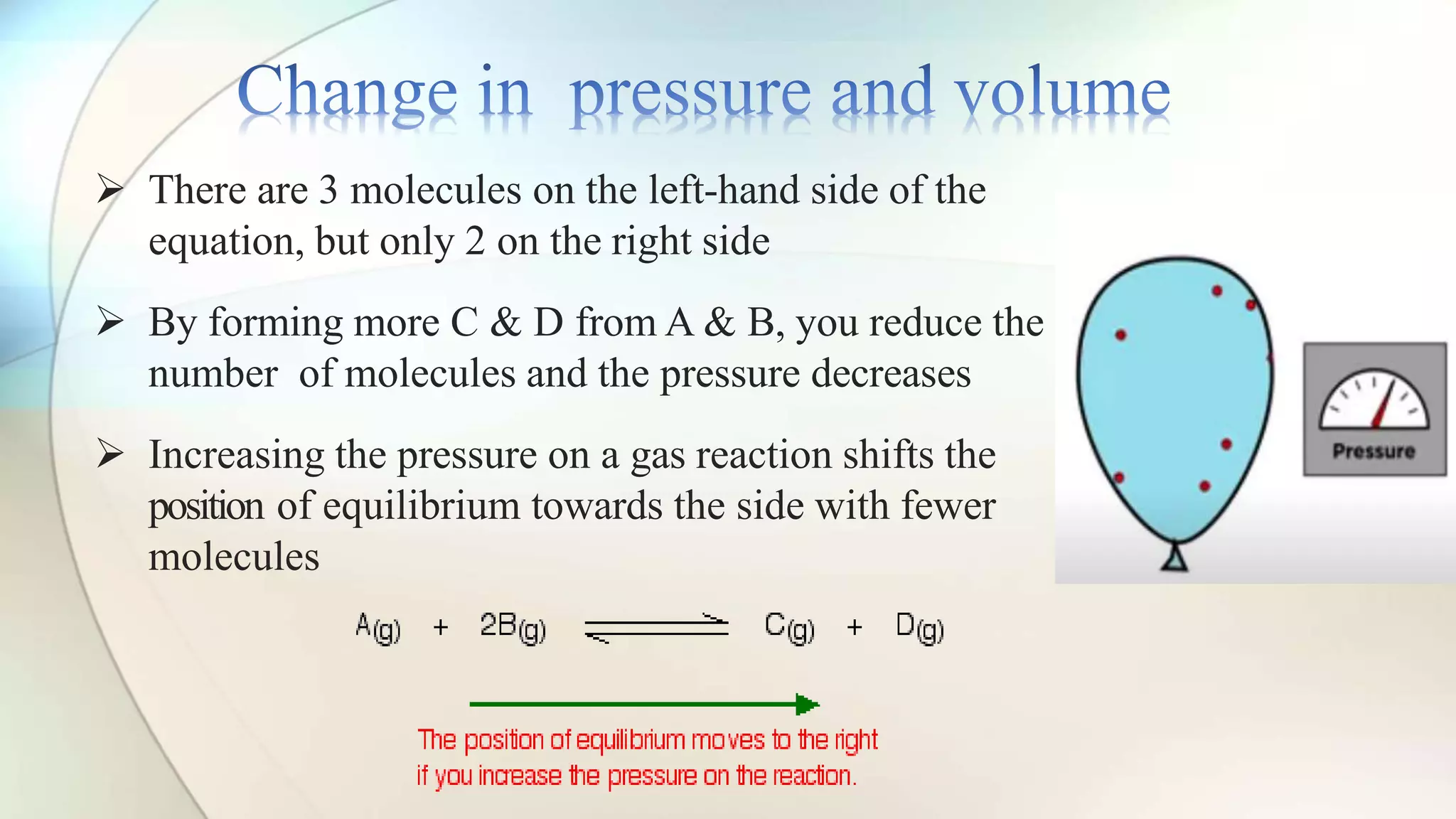
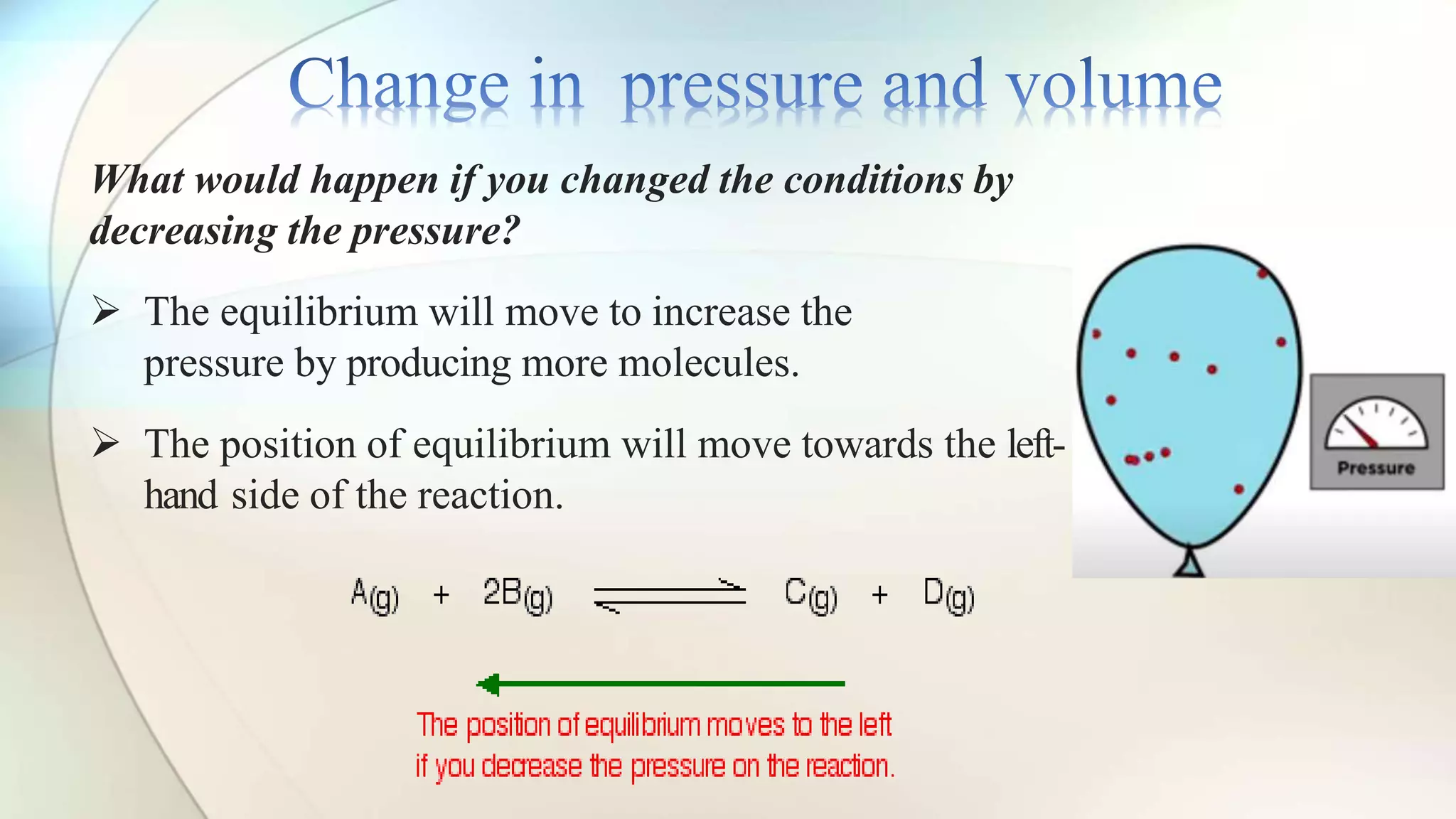

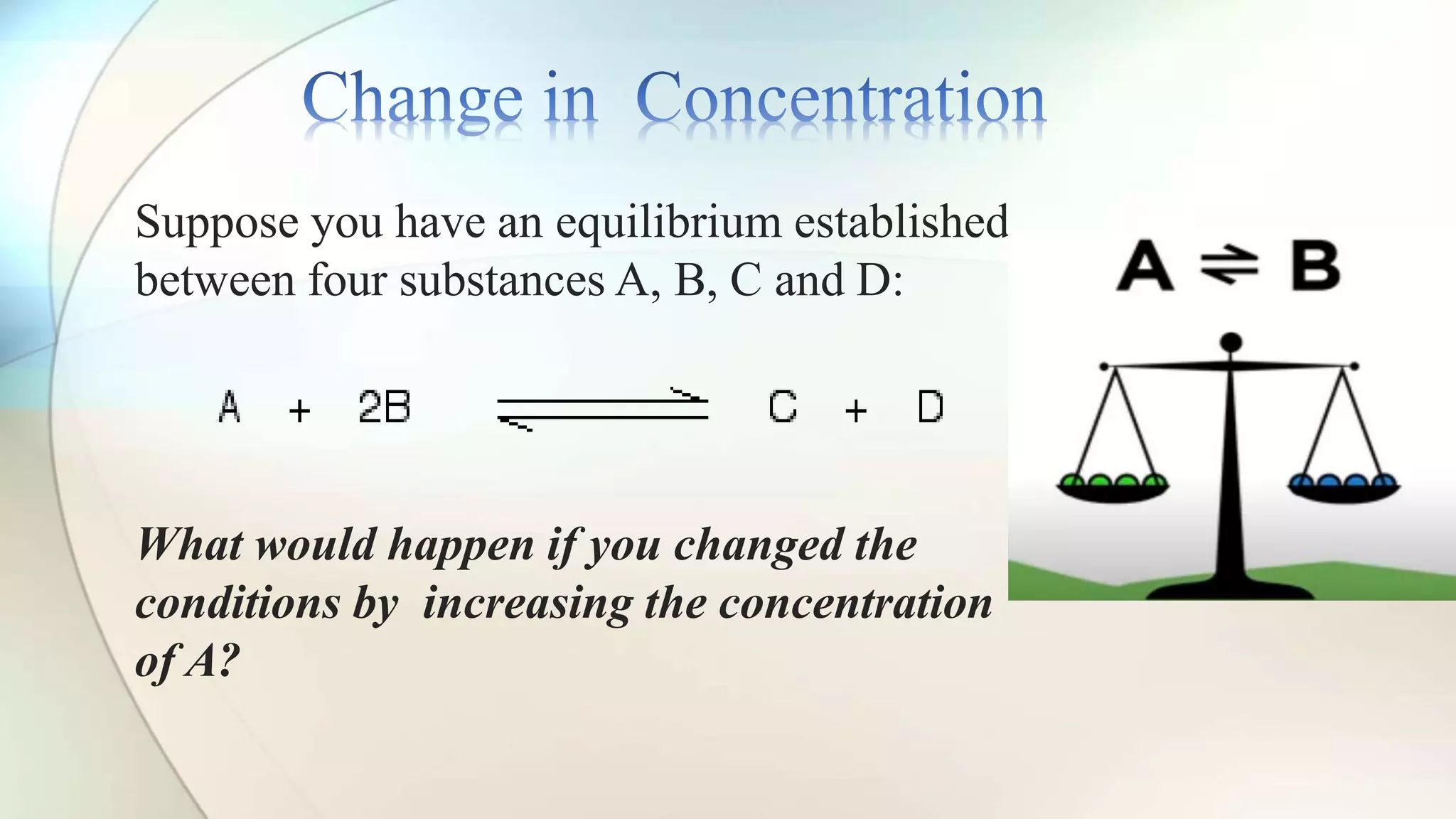
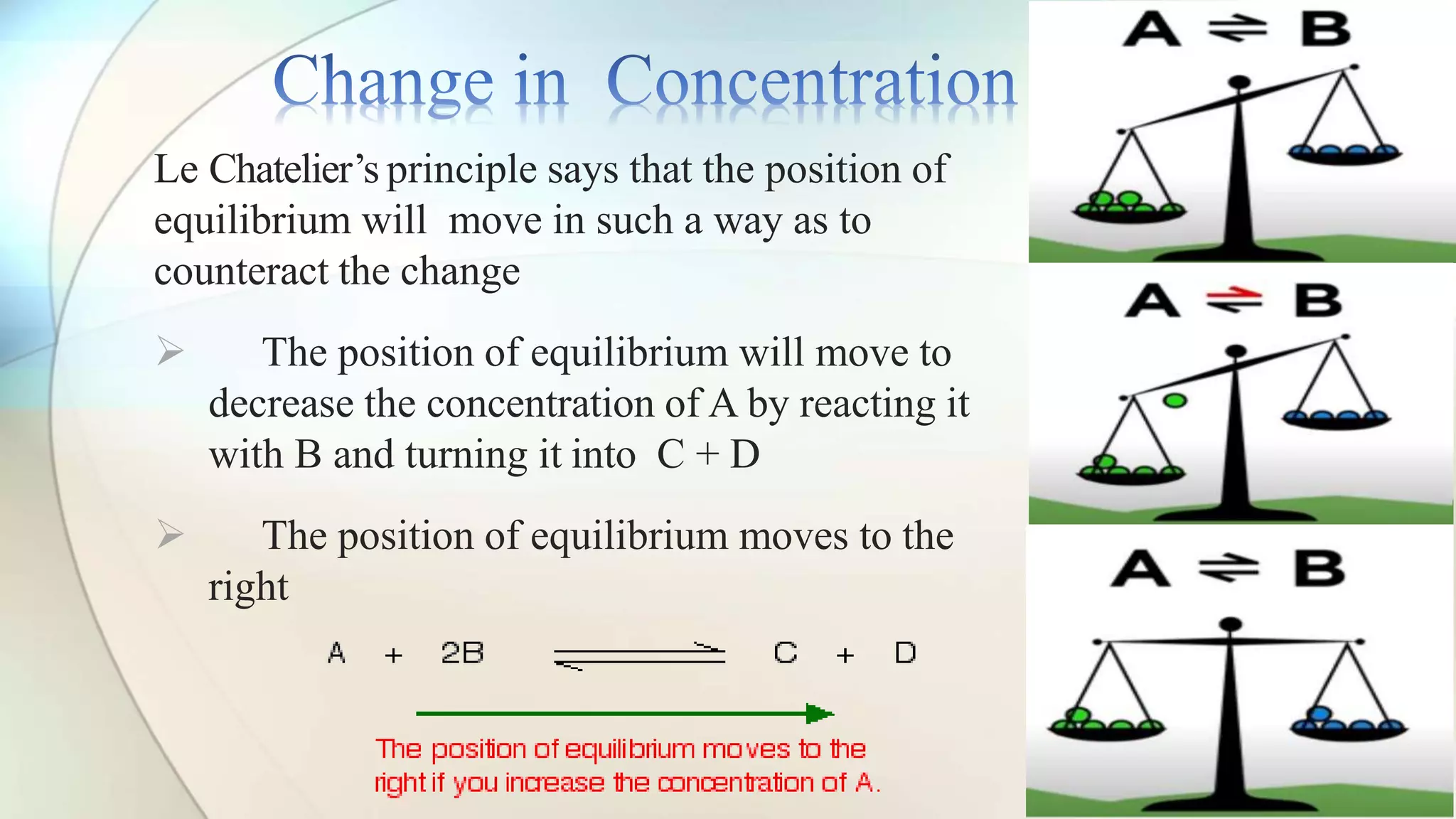
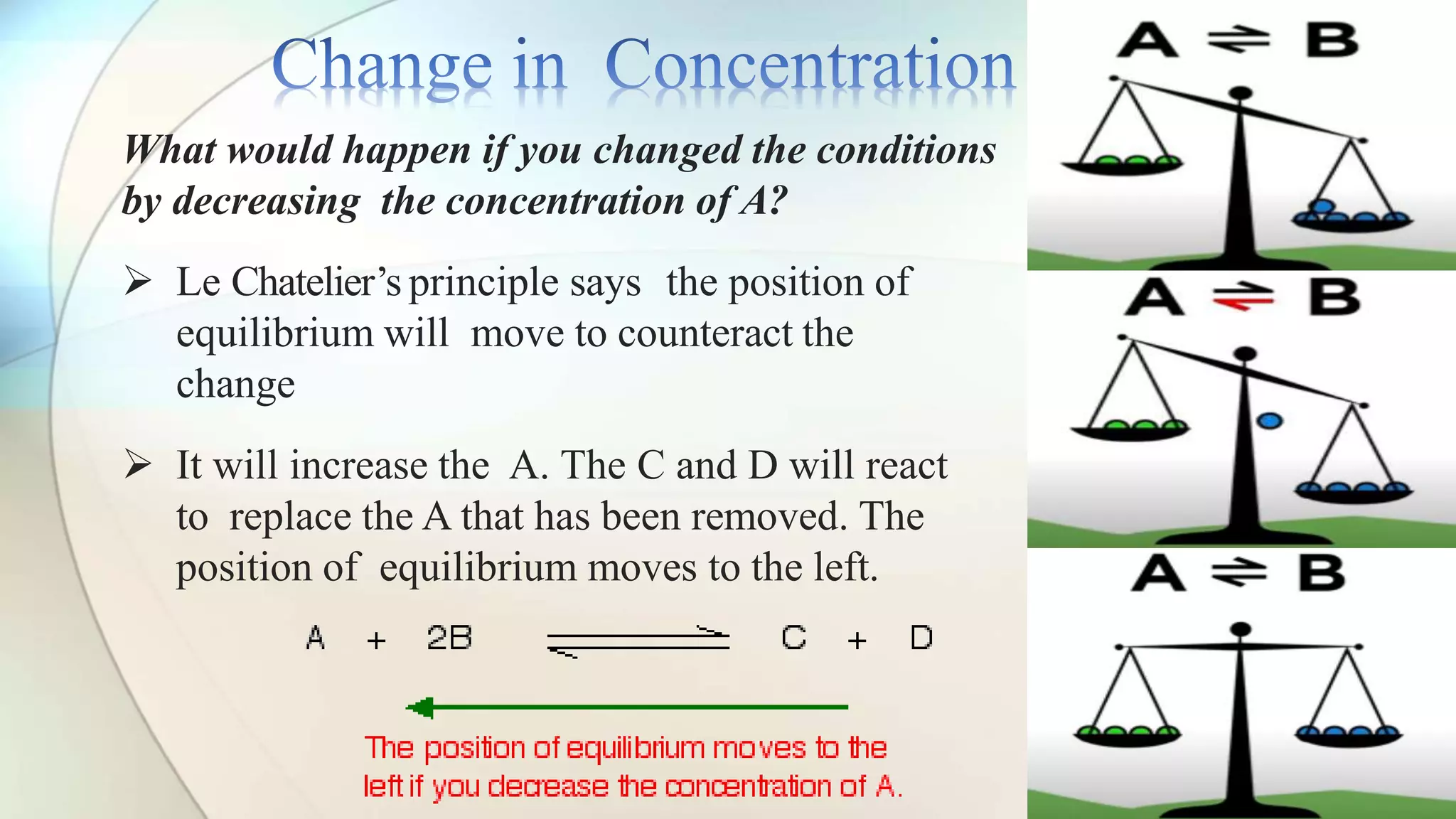
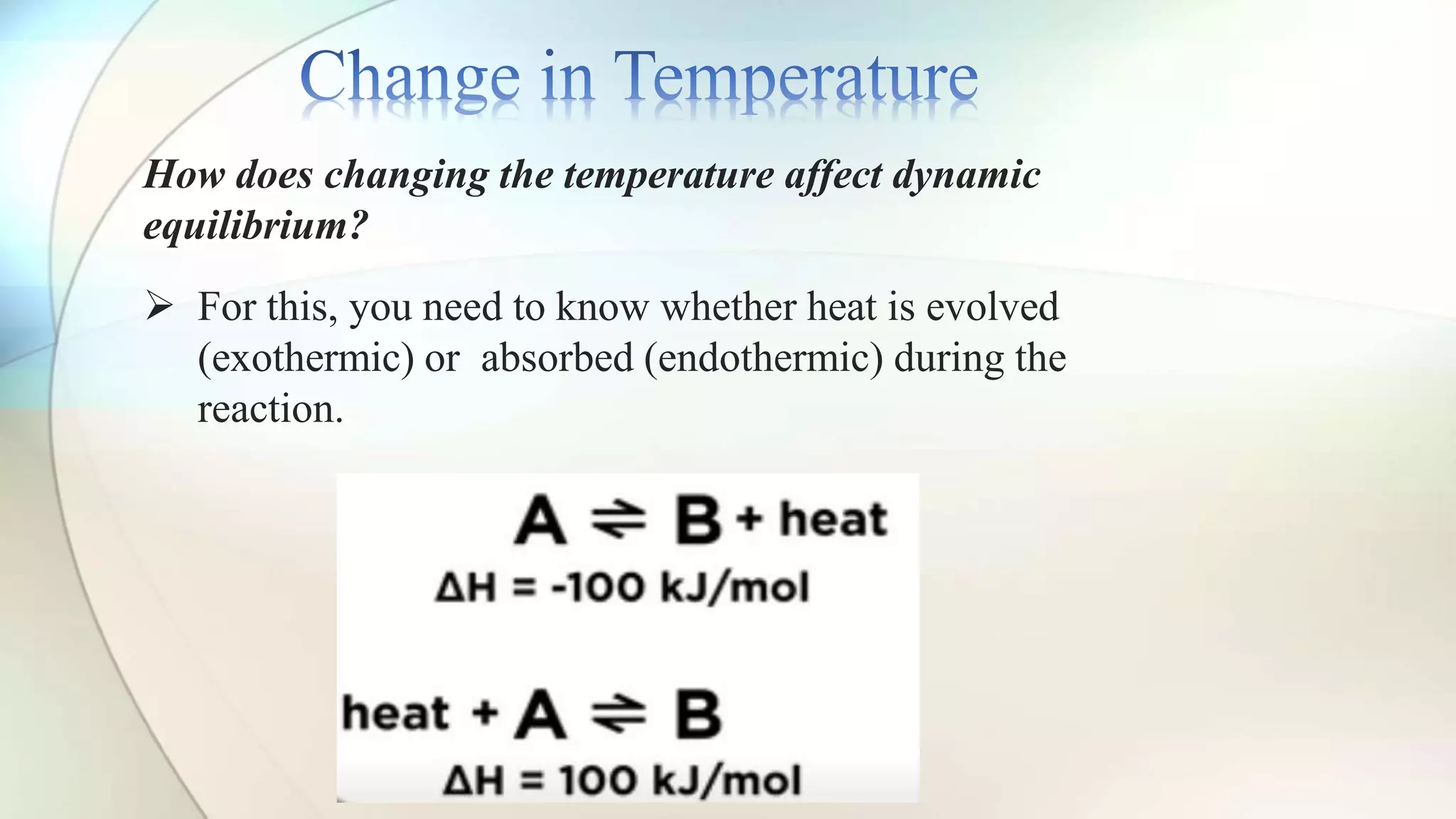

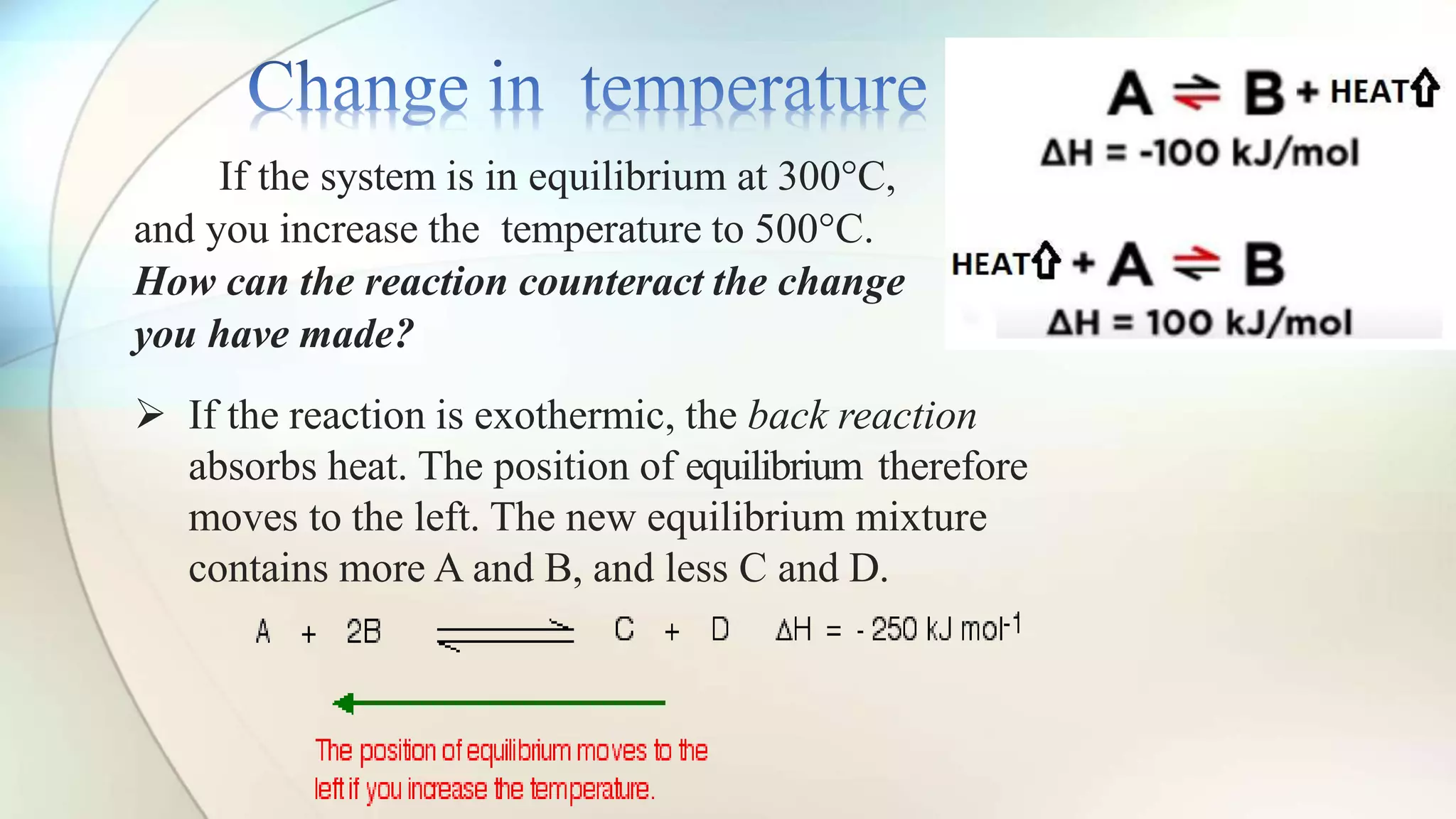

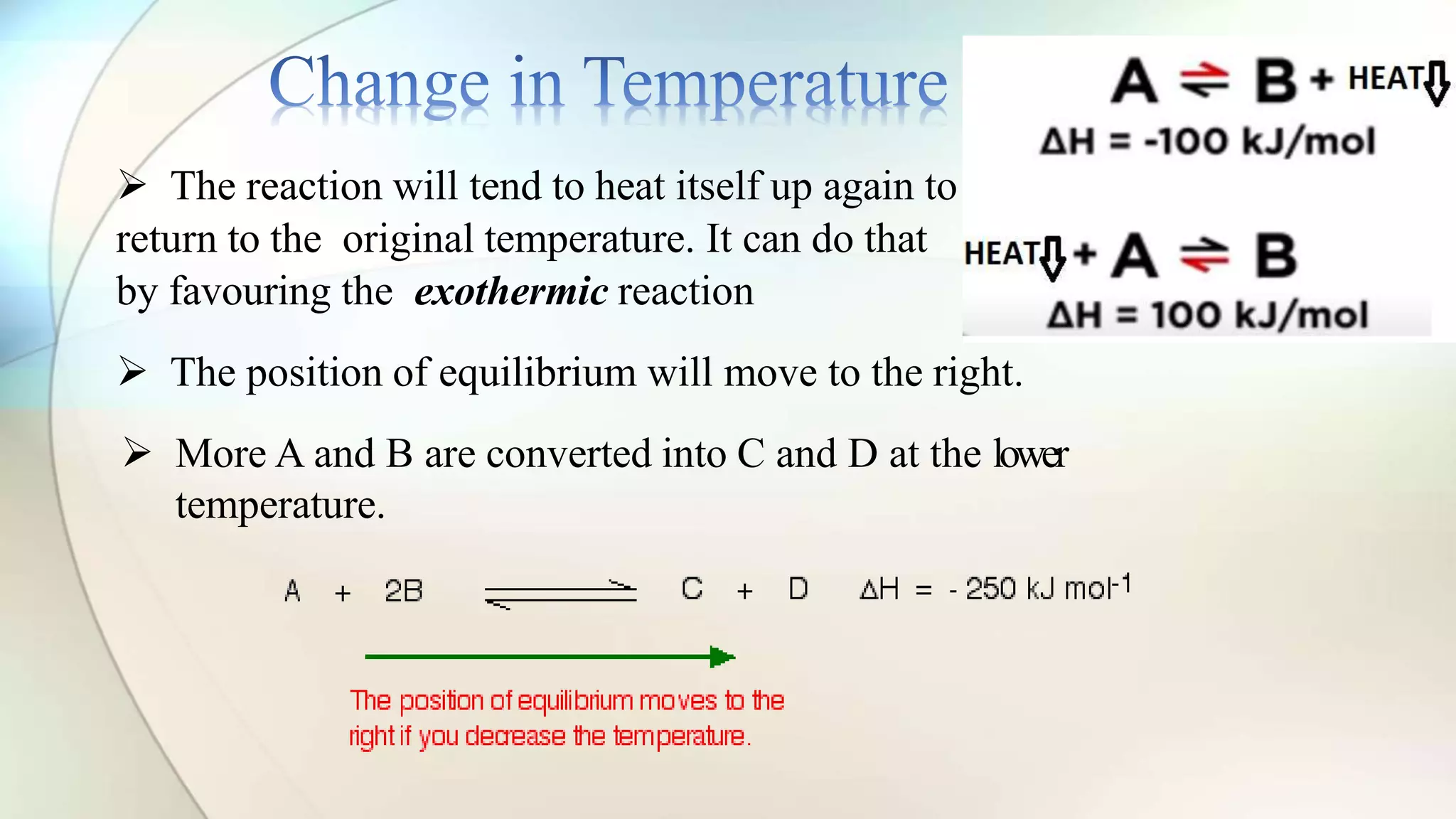




Le Chatelier's principle states that a system at equilibrium will adjust to counteract changes in concentration, temperature, or pressure. Increasing pressure shifts the equilibrium towards the side with fewer gas molecules, while decreasing pressure moves it towards the side with more molecules. Additionally, changes in temperature affect equilibrium based on whether the reaction is exothermic or endothermic, shifting accordingly to maintain balance.

















