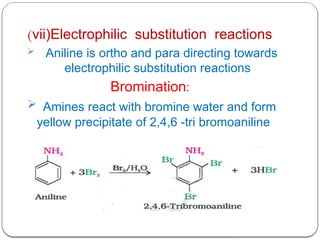
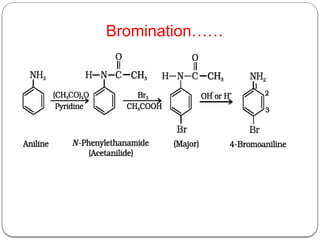
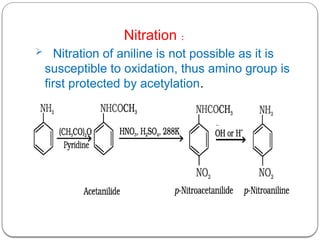
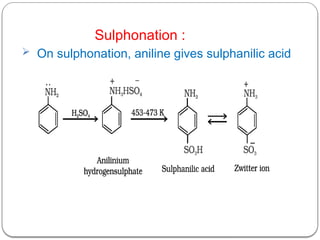
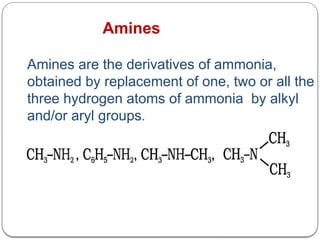
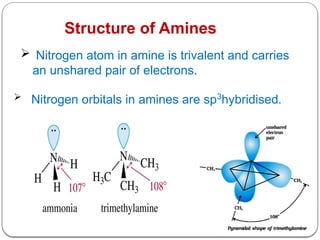
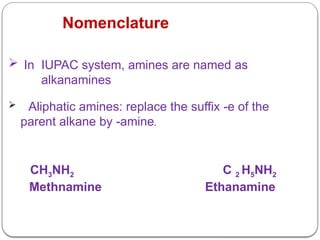
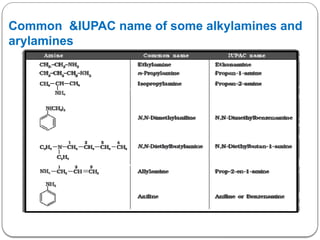
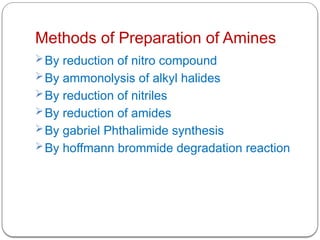
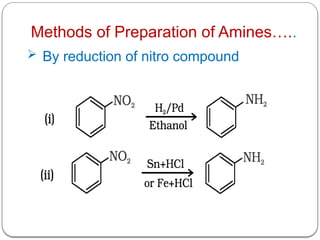
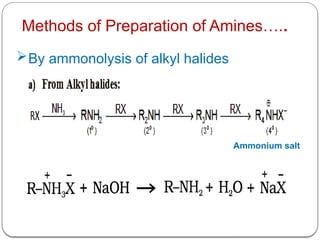
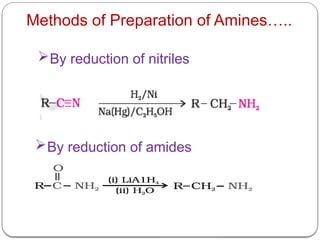
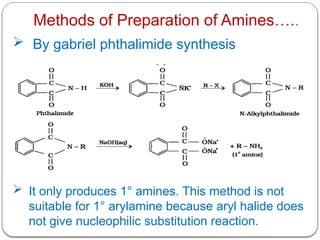
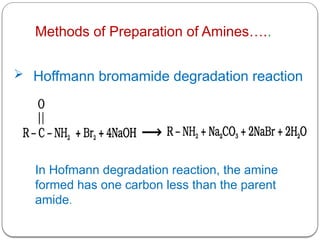
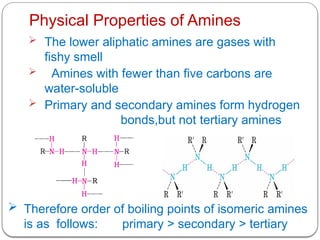
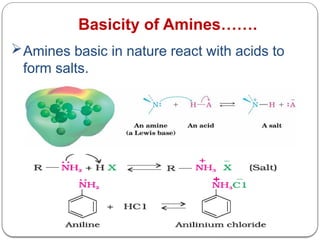
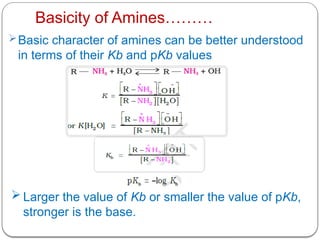
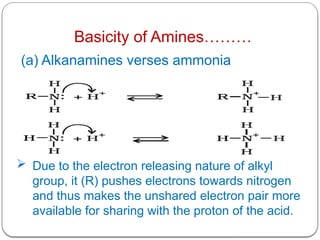
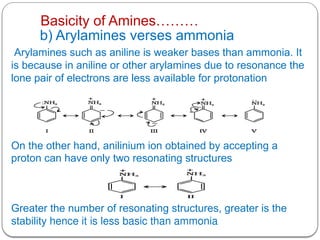
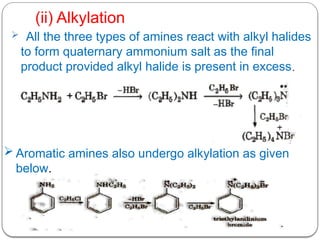
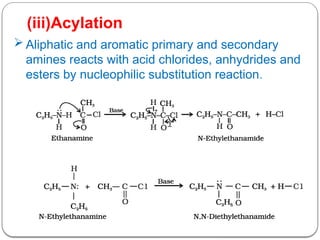
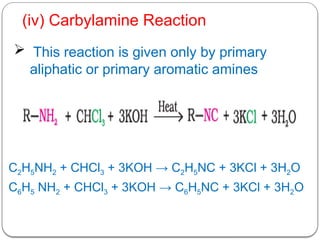
This document outlines the study of organic compounds containing nitrogen, focusing specifically on amines. It covers their structure, classification, nomenclature, methods of preparation, and physical and chemical properties. The document emphasizes the basicity of amines and includes specific examples and reactions, such as alkylation and acylation.


![(vi)Reaction with benzensulphonyl chloride
[Hinsberg reagent]
The reaction of benzenesulphonyl chloride with
primary amine yield N-ethyl benzenesulphonyl
amide.
Tertiary amines does not react with benzenesulphonyl
chloride.](https://image.slidesharecdn.com/13amines1-241208140633-55f5b766/85/1313-Amines-1-pptx-27-320.jpg)